 Found 308 hits with Last Name = 'poel' and Initial = 'tj'
Found 308 hits with Last Name = 'poel' and Initial = 'tj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50116067
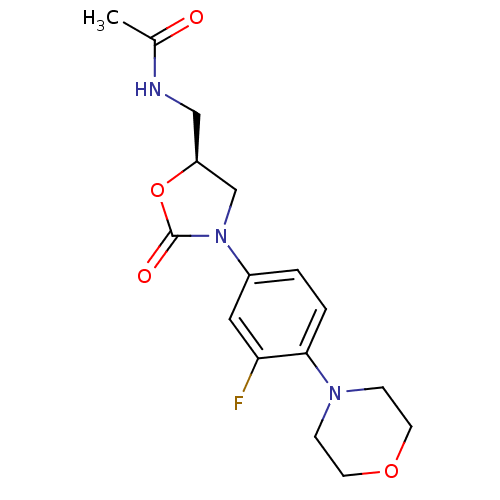
((Linezolid)N-[3-(3-Fluoro-4-morpholin-4-yl-phenyl)...)Show SMILES CC(=O)NC[C@H]1CN(C(=O)O1)c1ccc(N2CCOCC2)c(F)c1 |r| Show InChI InChI=1S/C16H20FN3O4/c1-11(21)18-9-13-10-20(16(22)24-13)12-2-3-15(14(17)8-12)19-4-6-23-7-5-19/h2-3,8,13H,4-7,9-10H2,1H3,(H,18,21)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226485

((R)-3-(3-fluoro-4-(tetrahydro-2H-pyran-4-yl)phenyl...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1ccc(C2CCOCC2)c(F)c1 Show InChI InChI=1S/C15H17FN2O4/c16-12-7-10(18-8-13(14(17)19)22-15(18)20)1-2-11(12)9-3-5-21-6-4-9/h1-2,7,9,13H,3-6,8H2,(H2,17,19)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226479
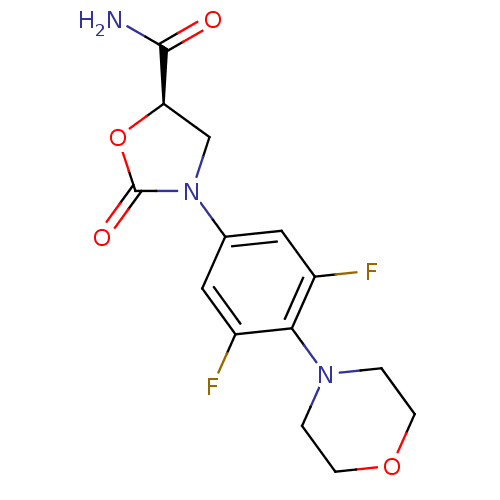
((R)-3-(3,5-difluoro-4-morpholinophenyl)-2-oxooxazo...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1cc(F)c(N2CCOCC2)c(F)c1 Show InChI InChI=1S/C14H15F2N3O4/c15-9-5-8(19-7-11(13(17)20)23-14(19)21)6-10(16)12(9)18-1-3-22-4-2-18/h5-6,11H,1-4,7H2,(H2,17,20)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226484
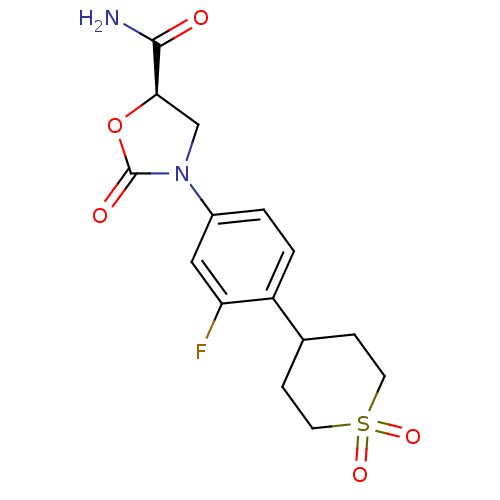
((5R)-3-[4-(1,1-dioxidotetrahydro-2H-thiopyran-4-yl...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1ccc(C2CCS(=O)(=O)CC2)c(F)c1 Show InChI InChI=1S/C15H17FN2O5S/c16-12-7-10(18-8-13(14(17)19)23-15(18)20)1-2-11(12)9-3-5-24(21,22)6-4-9/h1-2,7,9,13H,3-6,8H2,(H2,17,19)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226480
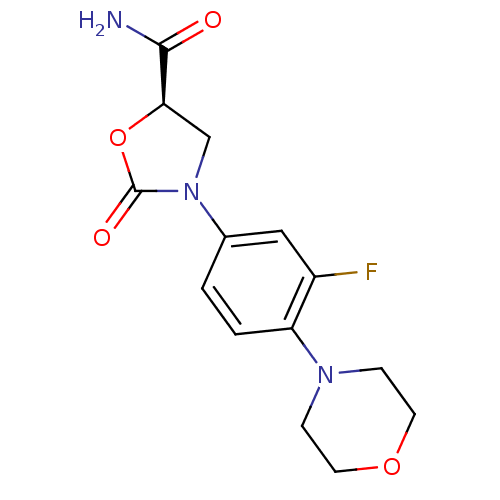
((R)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidi...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1ccc(N2CCOCC2)c(F)c1 Show InChI InChI=1S/C14H16FN3O4/c15-10-7-9(18-8-12(13(16)19)22-14(18)20)1-2-11(10)17-3-5-21-6-4-17/h1-2,7,12H,3-6,8H2,(H2,16,19)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226481
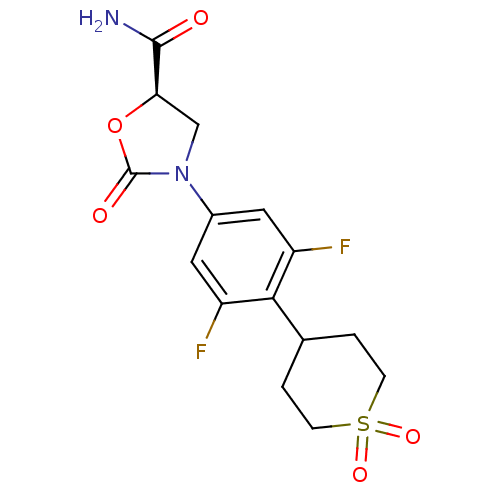
((5R)-3-[4-(1,1-dioxidotetrahydro-2H-thiopyran-4-yl...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1cc(F)c(C2CCS(=O)(=O)CC2)c(F)c1 Show InChI InChI=1S/C15H16F2N2O5S/c16-10-5-9(19-7-12(14(18)20)24-15(19)21)6-11(17)13(10)8-1-3-25(22,23)4-2-8/h5-6,8,12H,1-4,7H2,(H2,18,20)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50226482
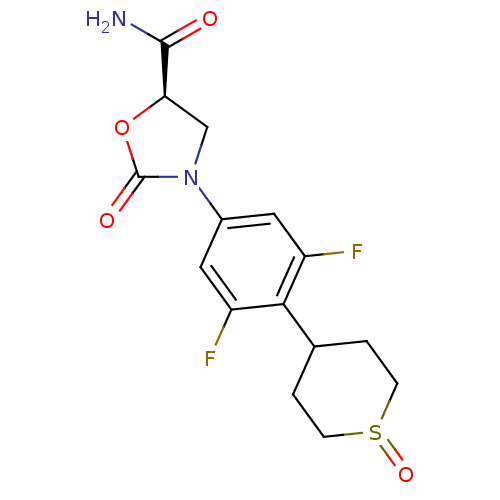
((5R)-3-[3,5-difluoro-4-(1-oxidotetrahydro-2H-thiop...)Show SMILES NC(=O)[C@H]1CN(C(=O)O1)c1cc(F)c(C2CCS(=O)CC2)c(F)c1 Show InChI InChI=1S/C15H16F2N2O4S/c16-10-5-9(19-7-12(14(18)20)23-15(19)21)6-11(17)13(10)8-1-3-24(22)4-2-8/h5-6,8,12H,1-4,7H2,(H2,18,20)/t8?,12-,24?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 50: 5886-9 (2007)
Article DOI: 10.1021/jm070708p
BindingDB Entry DOI: 10.7270/Q22J6BM7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215692

(CHEMBL400874 | sodium (3R,5R)-7-(5-((4-cyanobenzyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ccc(cc1)C#N)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C35H36FN3O5/c1-22(2)39-30(17-16-28(40)18-29(41)19-31(42)43)32(26-12-14-27(36)15-13-26)33(25-6-4-3-5-7-25)34(39)35(44)38-21-24-10-8-23(20-37)9-11-24/h3-15,22,28-29,40-41H,16-19,21H2,1-2H3,(H,38,44)(H,42,43)/p-1/t28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215693
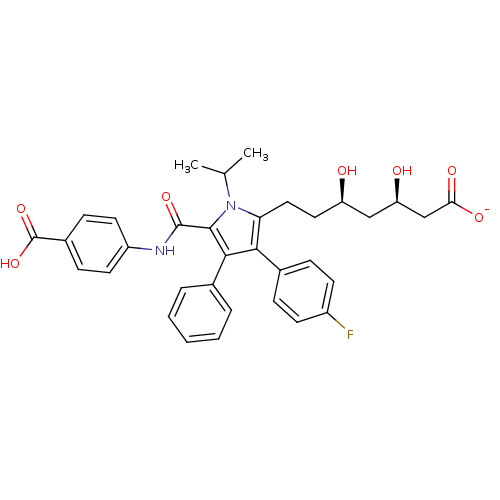
(CHEMBL437774 | sodium (3R,5R)-7-(5-((4-carboxyphen...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccc(cc1)C(O)=O)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H35FN2O7/c1-20(2)37-28(17-16-26(38)18-27(39)19-29(40)41)30(22-8-12-24(35)13-9-22)31(21-6-4-3-5-7-21)32(37)33(42)36-25-14-10-23(11-15-25)34(43)44/h3-15,20,26-27,38-39H,16-19H2,1-2H3,(H,36,42)(H,40,41)(H,43,44)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215688
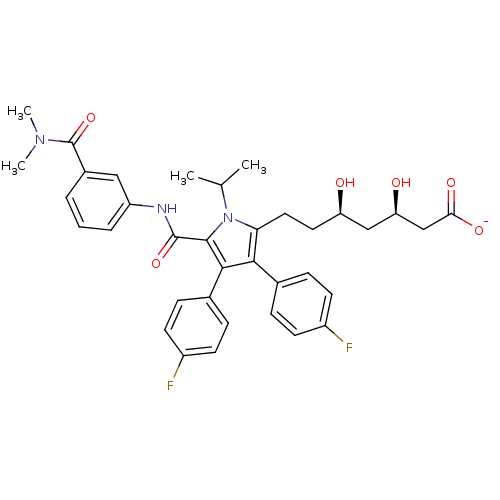
(CHEMBL399773 | sodium (3R,5R)-7-(5-((3-(dimethylca...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1cccc(c1)C(=O)N(C)C)-c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C36H39F2N3O6/c1-21(2)41-30(17-16-28(42)19-29(43)20-31(44)45)32(22-8-12-25(37)13-9-22)33(23-10-14-26(38)15-11-23)34(41)35(46)39-27-7-5-6-24(18-27)36(47)40(3)4/h5-15,18,21,28-29,42-43H,16-17,19-20H2,1-4H3,(H,39,46)(H,44,45)/p-1/t28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215689

(CHEMBL250317 | sodium (3R,5R)-7-(5-((4-carbamoylph...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccc(cc1)C(N)=O)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H36FN3O6/c1-20(2)38-28(17-16-26(39)18-27(40)19-29(41)42)30(22-8-12-24(35)13-9-22)31(21-6-4-3-5-7-21)32(38)34(44)37-25-14-10-23(11-15-25)33(36)43/h3-15,20,26-27,39-40H,16-19H2,1-2H3,(H2,36,43)(H,37,44)(H,41,42)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215700

(CHEMBL400973 | sodium (3R,5R)-7-(3-(4-fluorophenyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccc(O)cc1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H35FN2O6/c1-20(2)36-28(17-16-26(38)18-27(39)19-29(40)41)30(22-8-10-23(34)11-9-22)31(21-6-4-3-5-7-21)32(36)33(42)35-24-12-14-25(37)15-13-24/h3-15,20,26-27,37-39H,16-19H2,1-2H3,(H,35,42)(H,40,41)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215704

(CHEMBL398551 | sodium (3R,5R)-7-(5-(((1,5-dimethyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1cc(C)n(C)n1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H39FN4O5/c1-20(2)38-28(15-14-26(39)17-27(40)18-29(41)42)30(23-10-12-24(34)13-11-23)31(22-8-6-5-7-9-22)32(38)33(43)35-19-25-16-21(3)37(4)36-25/h5-13,16,20,26-27,39-40H,14-15,17-19H2,1-4H3,(H,35,43)(H,41,42)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215699

(CHEMBL400747 | sodium (3R,5R)-7-(3,4-bis(4-fluorop...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1cccc(O)c1)-c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H34F2N2O6/c1-19(2)37-28(15-14-26(39)17-27(40)18-29(41)42)30(20-6-10-22(34)11-7-20)31(21-8-12-23(35)13-9-21)32(37)33(43)36-24-4-3-5-25(38)16-24/h3-13,16,19,26-27,38-40H,14-15,17-18H2,1-2H3,(H,36,43)(H,41,42)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215707
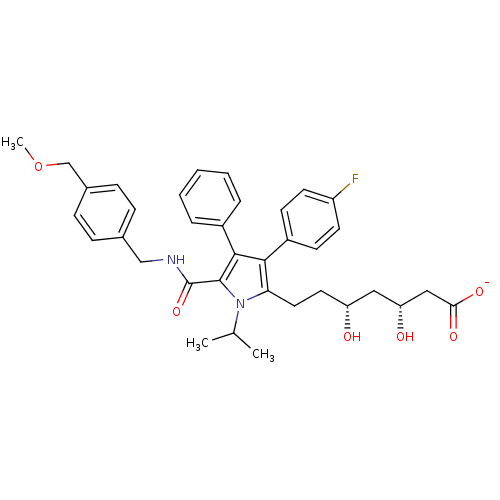
(CHEMBL250749 | sodium (3R,5R)-7-(5-((4-(methoxymet...)Show SMILES COCc1ccc(CNC(=O)c2c(c(c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)n2C(C)C)-c2ccc(F)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C36H41FN2O6/c1-23(2)39-31(18-17-29(40)19-30(41)20-32(42)43)33(27-13-15-28(37)16-14-27)34(26-7-5-4-6-8-26)35(39)36(44)38-21-24-9-11-25(12-10-24)22-45-3/h4-16,23,29-30,40-41H,17-22H2,1-3H3,(H,38,44)(H,42,43)/p-1/t29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215690

(CHEMBL399313 | sodium (3R,5R)-7-(5-(benzylcarbamoy...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ccccc1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H37FN2O5/c1-22(2)37-29(18-17-27(38)19-28(39)20-30(40)41)31(25-13-15-26(35)16-14-25)32(24-11-7-4-8-12-24)33(37)34(42)36-21-23-9-5-3-6-10-23/h3-16,22,27-28,38-39H,17-21H2,1-2H3,(H,36,42)(H,40,41)/p-1/t27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215708
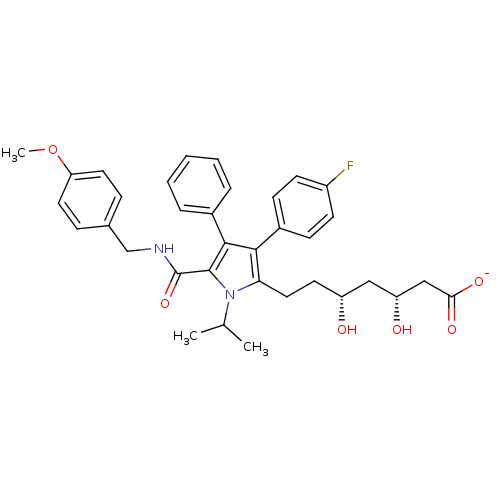
(CHEMBL249724 | sodium (3R,5R)-7-(5-((4-methoxybenz...)Show SMILES COc1ccc(CNC(=O)c2c(c(c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)n2C(C)C)-c2ccc(F)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C35H39FN2O6/c1-22(2)38-30(18-15-27(39)19-28(40)20-31(41)42)32(25-11-13-26(36)14-12-25)33(24-7-5-4-6-8-24)34(38)35(43)37-21-23-9-16-29(44-3)17-10-23/h4-14,16-17,22,27-28,39-40H,15,18-21H2,1-3H3,(H,37,43)(H,41,42)/p-1/t27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215684
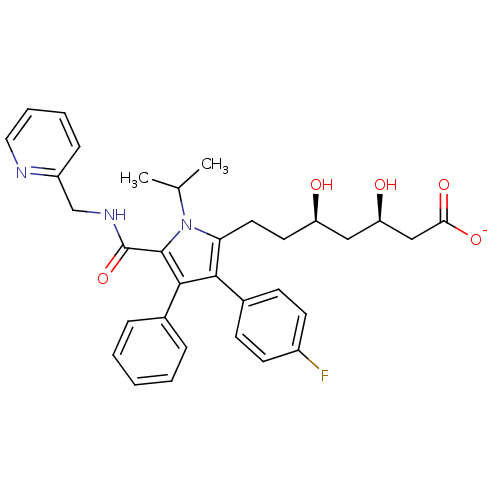
(CHEMBL398240 | sodium (3R,5R)-7-(3-(4-fluorophenyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ccccn1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H36FN3O5/c1-21(2)37-28(16-15-26(38)18-27(39)19-29(40)41)30(23-11-13-24(34)14-12-23)31(22-8-4-3-5-9-22)32(37)33(42)36-20-25-10-6-7-17-35-25/h3-14,17,21,26-27,38-39H,15-16,18-20H2,1-2H3,(H,36,42)(H,40,41)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215694
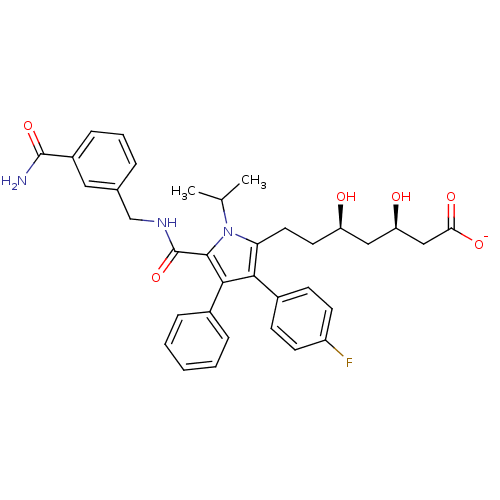
(CHEMBL249906 | sodium (3R,5R)-7-(5-((3-carbamoylbe...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1cccc(c1)C(N)=O)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C35H38FN3O6/c1-21(2)39-29(16-15-27(40)18-28(41)19-30(42)43)31(24-11-13-26(36)14-12-24)32(23-8-4-3-5-9-23)33(39)35(45)38-20-22-7-6-10-25(17-22)34(37)44/h3-14,17,21,27-28,40-41H,15-16,18-20H2,1-2H3,(H2,37,44)(H,38,45)(H,42,43)/p-1/t27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215698

(CHEMBL251499 | sodium (3R,5R)-7-(5-((4-(dimethylca...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ccc(cc1)C(=O)N(C)C)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C37H42FN3O6/c1-23(2)41-31(19-18-29(42)20-30(43)21-32(44)45)33(26-14-16-28(38)17-15-26)34(25-8-6-5-7-9-25)35(41)36(46)39-22-24-10-12-27(13-11-24)37(47)40(3)4/h5-17,23,29-30,42-43H,18-22H2,1-4H3,(H,39,46)(H,44,45)/p-1/t29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215683
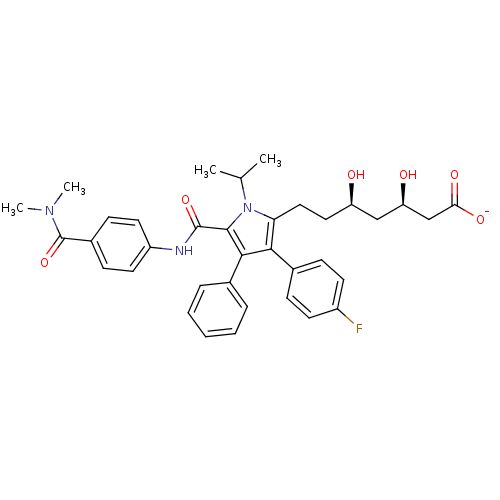
(CHEMBL403127 | sodium (3R,5R)-7-(5-((4-(dimethylca...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccc(cc1)C(=O)N(C)C)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C36H40FN3O6/c1-22(2)40-30(19-18-28(41)20-29(42)21-31(43)44)32(24-10-14-26(37)15-11-24)33(23-8-6-5-7-9-23)34(40)35(45)38-27-16-12-25(13-17-27)36(46)39(3)4/h5-17,22,28-29,41-42H,18-21H2,1-4H3,(H,38,45)(H,43,44)/p-1/t28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215691
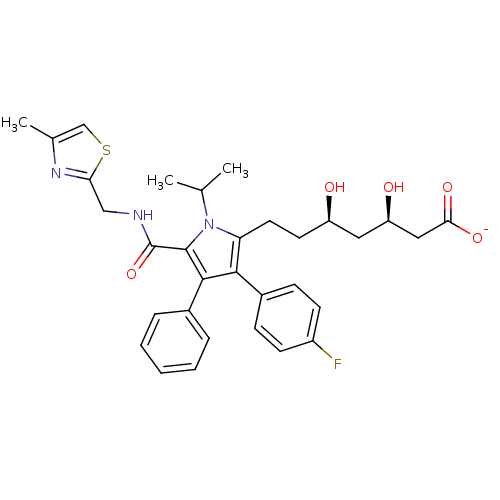
(CHEMBL398239 | sodium (3R,5R)-7-(3-(4-fluorophenyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1nc(C)cs1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H36FN3O5S/c1-19(2)36-26(14-13-24(37)15-25(38)16-28(39)40)29(22-9-11-23(33)12-10-22)30(21-7-5-4-6-8-21)31(36)32(41)34-17-27-35-20(3)18-42-27/h4-12,18-19,24-25,37-38H,13-17H2,1-3H3,(H,34,41)(H,39,40)/p-1/t24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215706
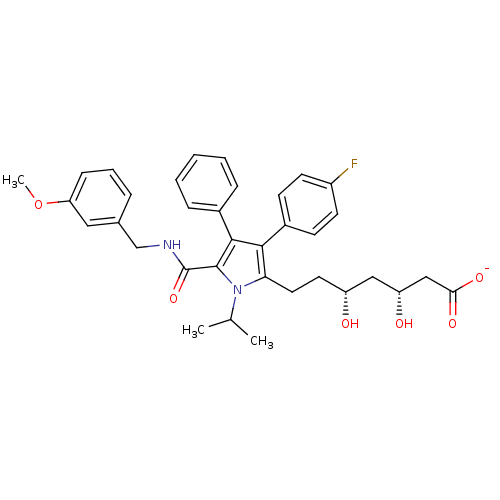
(CHEMBL399315 | sodium (3R,5R)-7-(5-((3-methoxybenz...)Show SMILES COc1cccc(CNC(=O)c2c(c(c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)n2C(C)C)-c2ccc(F)cc2)-c2ccccc2)c1 Show InChI InChI=1S/C35H39FN2O6/c1-22(2)38-30(17-16-27(39)19-28(40)20-31(41)42)32(25-12-14-26(36)15-13-25)33(24-9-5-4-6-10-24)34(38)35(43)37-21-23-8-7-11-29(18-23)44-3/h4-15,18,22,27-28,39-40H,16-17,19-21H2,1-3H3,(H,37,43)(H,41,42)/p-1/t27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM18374
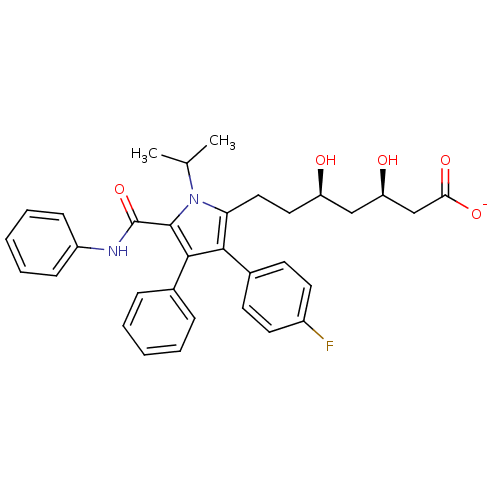
(CHEMBL394937 | Pyrrole-based compound, 30 | sodium...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccccc1)-c1ccccc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C33H35FN2O5/c1-21(2)36-28(18-17-26(37)19-27(38)20-29(39)40)30(23-13-15-24(34)16-14-23)31(22-9-5-3-6-10-22)32(36)33(41)35-25-11-7-4-8-12-25/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18390

((3R,5S)-6-[(3-{[(2,3-difluorophenyl)methyl]carbamo...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1cccc(F)c1F)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H28F3N3O6/c1-14(2)22-24(25(37)30-12-15-4-3-5-20(28)23(15)29)31-32(17-8-6-16(27)7-9-17)26(22)38-13-19(34)10-18(33)11-21(35)36/h3-9,14,18-19,33-34H,10-13H2,1-2H3,(H,30,37)(H,35,36)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18427

((3R,5R)-7-[1-(4-fluorophenyl)-3-{[(3R)-3-phenylpip...)Show SMILES CC(C)c1c(CC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)N1CCC[C@@H](C1)c1ccccc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H38FN3O5/c1-20(2)29-27(15-14-25(36)17-26(37)18-28(38)39)35(24-12-10-23(32)11-13-24)33-30(29)31(40)34-16-6-9-22(19-34)21-7-4-3-5-8-21/h3-5,7-8,10-13,20,22,25-26,36-37H,6,9,14-19H2,1-2H3,(H,38,39)/t22-,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18372
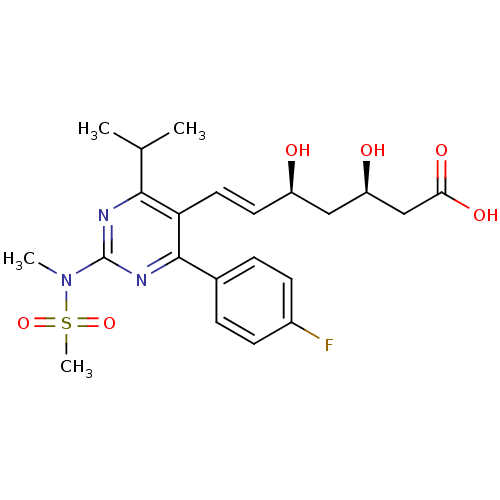
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215686

(CHEMBL401293 | sodium (3R,5R)-7-(5-((4-carboxybenz...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ccc(cc1)C(O)=O)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C35H37FN2O7/c1-21(2)38-29(17-16-27(39)18-28(40)19-30(41)42)31(24-12-14-26(36)15-13-24)32(23-6-4-3-5-7-23)33(38)34(43)37-20-22-8-10-25(11-9-22)35(44)45/h3-15,21,27-28,39-40H,16-20H2,1-2H3,(H,37,43)(H,41,42)(H,44,45)/p-1/t27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18425
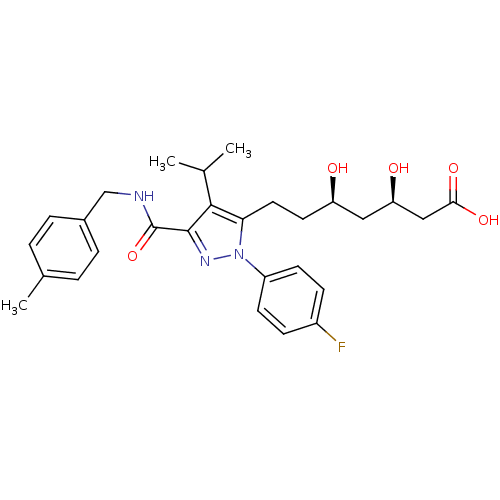
((3R,5R)-7-[1-(4-fluorophenyl)-3-{[(4-methylphenyl)...)Show SMILES CC(C)c1c(CC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1ccc(C)cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C28H34FN3O5/c1-17(2)26-24(13-12-22(33)14-23(34)15-25(35)36)32(21-10-8-20(29)9-11-21)31-27(26)28(37)30-16-19-6-4-18(3)5-7-19/h4-11,17,22-23,33-34H,12-16H2,1-3H3,(H,30,37)(H,35,36)/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215696

(CHEMBL400560 | sodium (3R,5R)-7-(3-(4-fluorophenyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1cc(C)n[nH]1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H37FN4O5/c1-19(2)37-27(14-13-25(38)16-26(39)17-28(40)41)29(22-9-11-23(33)12-10-22)30(21-7-5-4-6-8-21)31(37)32(42)34-18-24-15-20(3)35-36-24/h4-12,15,19,25-26,38-39H,13-14,16-18H2,1-3H3,(H,34,42)(H,35,36)(H,40,41)/p-1/t25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215680
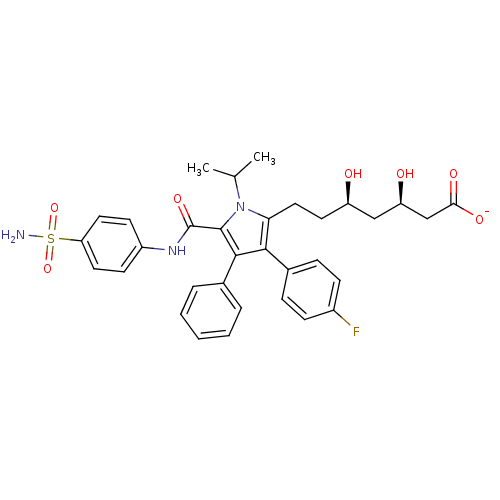
(CHEMBL250953 | sodium (3R,5R)-7-[3-(4-fluoro-pheny...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccc(cc1)S(N)(=O)=O)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H36FN3O7S/c1-20(2)37-28(17-14-25(38)18-26(39)19-29(40)41)30(22-8-10-23(34)11-9-22)31(21-6-4-3-5-7-21)32(37)33(42)36-24-12-15-27(16-13-24)45(35,43)44/h3-13,15-16,20,25-26,38-39H,14,17-19H2,1-2H3,(H,36,42)(H,40,41)(H2,35,43,44)/p-1/t25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215687
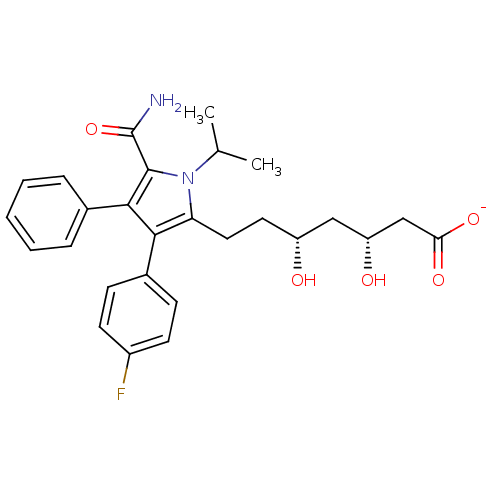
(CHEMBL438694 | sodium (3R,5R)-7-(5-carbamoyl-3-(4-...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(N)=O)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H31FN2O5/c1-16(2)30-22(13-12-20(31)14-21(32)15-23(33)34)24(18-8-10-19(28)11-9-18)25(26(30)27(29)35)17-6-4-3-5-7-17/h3-11,16,20-21,31-32H,12-15H2,1-2H3,(H2,29,35)(H,33,34)/p-1/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM18372

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215685

(CHEMBL249273 | sodium (3R,5R)-7-(3-(4-fluorophenyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ncc(C)[nH]1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H37FN4O5/c1-19(2)37-26(14-13-24(38)15-25(39)16-28(40)41)29(22-9-11-23(33)12-10-22)30(21-7-5-4-6-8-21)31(37)32(42)35-18-27-34-17-20(3)36-27/h4-12,17,19,24-25,38-39H,13-16,18H2,1-3H3,(H,34,36)(H,35,42)(H,40,41)/p-1/t24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18388
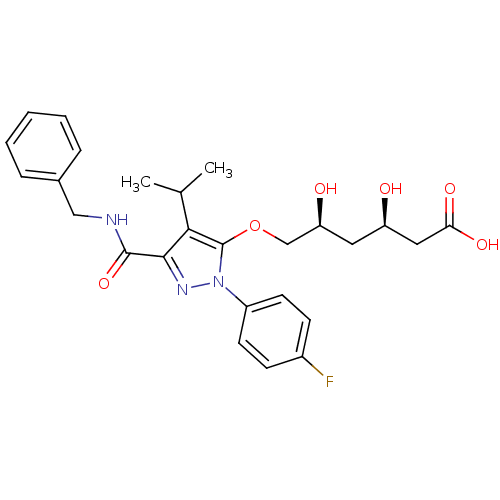
((3R,5S)-6-{[3-(benzylcarbamoyl)-1-(4-fluorophenyl)...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1ccccc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H30FN3O6/c1-16(2)23-24(25(35)28-14-17-6-4-3-5-7-17)29-30(19-10-8-18(27)9-11-19)26(23)36-15-21(32)12-20(31)13-22(33)34/h3-11,16,20-21,31-32H,12-15H2,1-2H3,(H,28,35)(H,33,34)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215682

(CHEMBL250707 | sodium (3R,5R)-7-(5-(cyclopropylcar...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NC1CC1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C30H35FN2O5/c1-18(2)33-25(15-14-23(34)16-24(35)17-26(36)37)27(20-8-10-21(31)11-9-20)28(19-6-4-3-5-7-19)29(33)30(38)32-22-12-13-22/h3-11,18,22-24,34-35H,12-17H2,1-2H3,(H,32,38)(H,36,37)/p-1/t23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
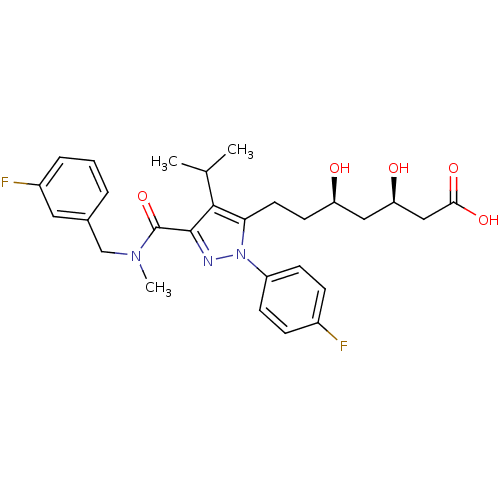
(Rattus norvegicus (rat)) | BDBM18426
((3R,5R)-7-[1-(4-fluorophenyl)-3-{[(3-fluorophenyl)...)Show SMILES CC(C)c1c(CC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)N(C)Cc1cccc(F)c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C28H33F2N3O5/c1-17(2)26-24(12-11-22(34)14-23(35)15-25(36)37)33(21-9-7-19(29)8-10-21)31-27(26)28(38)32(3)16-18-5-4-6-20(30)13-18/h4-10,13,17,22-23,34-35H,11-12,14-16H2,1-3H3,(H,36,37)/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18420

((3R,5S)-6-{[1-(4-fluorophenyl)-3-{[(3R)-3-phenylpi...)Show SMILES CC(C)c1c(nn(c1OC[C@@H](O)C[C@@H](O)CC(O)=O)-c1ccc(F)cc1)C(=O)N1CCC[C@@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C30H36FN3O6/c1-19(2)27-28(29(39)33-14-6-9-21(17-33)20-7-4-3-5-8-20)32-34(23-12-10-22(31)11-13-23)30(27)40-18-25(36)15-24(35)16-26(37)38/h3-5,7-8,10-13,19,21,24-25,35-36H,6,9,14-18H2,1-2H3,(H,37,38)/t21-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18394

((3R,5S)-6-{[1-(4-fluorophenyl)-3-{[(3-methylphenyl...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1cccc(C)c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H32FN3O6/c1-16(2)24-25(26(36)29-14-18-6-4-5-17(3)11-18)30-31(20-9-7-19(28)8-10-20)27(24)37-15-22(33)12-21(32)13-23(34)35/h4-11,16,21-22,32-33H,12-15H2,1-3H3,(H,29,36)(H,34,35)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18389
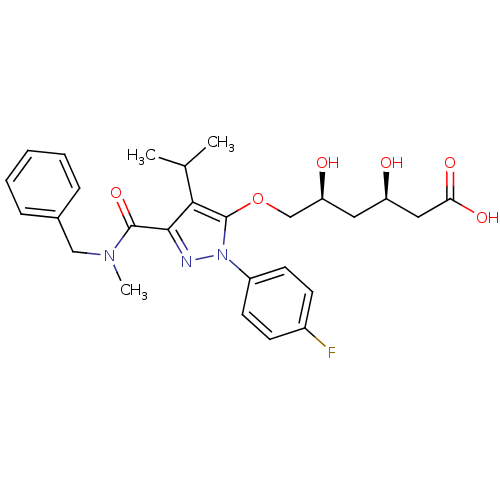
((3R,5S)-6-({3-[benzyl(methyl)carbamoyl]-1-(4-fluor...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)N(C)Cc1ccccc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H32FN3O6/c1-17(2)24-25(26(36)30(3)15-18-7-5-4-6-8-18)29-31(20-11-9-19(28)10-12-20)27(24)37-16-22(33)13-21(32)14-23(34)35/h4-12,17,21-22,32-33H,13-16H2,1-3H3,(H,34,35)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18400
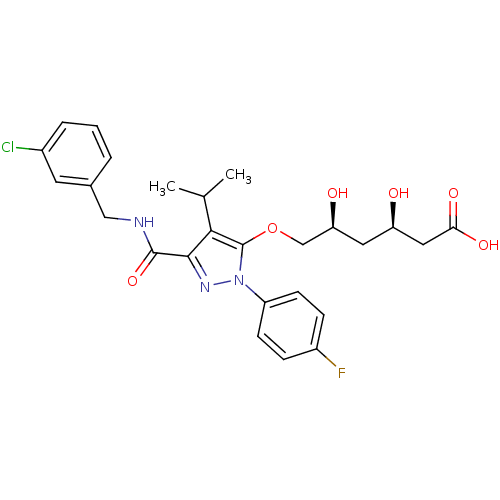
((3R,5S)-6-[(3-{[(3-chlorophenyl)methyl]carbamoyl}-...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1cccc(Cl)c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H29ClFN3O6/c1-15(2)23-24(25(36)29-13-16-4-3-5-17(27)10-16)30-31(19-8-6-18(28)7-9-19)26(23)37-14-21(33)11-20(32)12-22(34)35/h3-10,15,20-21,32-33H,11-14H2,1-2H3,(H,29,36)(H,34,35)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18409

((3R,5S)-6-[(3-{[(3,4-difluorophenyl)methyl](methyl...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)N(C)Cc1ccc(F)c(F)c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H30F3N3O6/c1-15(2)24-25(26(38)32(3)13-16-4-9-21(29)22(30)10-16)31-33(18-7-5-17(28)6-8-18)27(24)39-14-20(35)11-19(34)12-23(36)37/h4-10,15,19-20,34-35H,11-14H2,1-3H3,(H,36,37)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18424
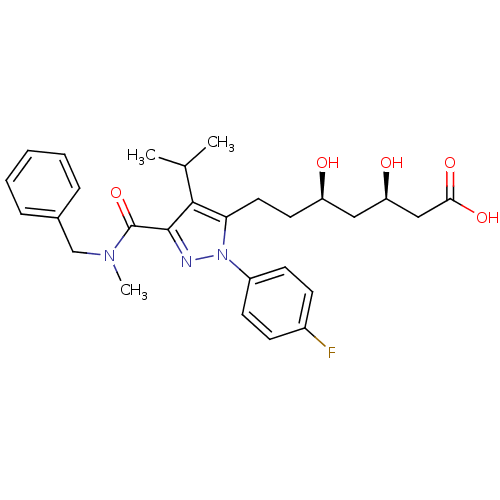
((3R,5R)-7-{3-[benzyl(methyl)carbamoyl]-1-(4-fluoro...)Show SMILES CC(C)c1c(CC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)N(C)Cc1ccccc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C28H34FN3O5/c1-18(2)26-24(14-13-22(33)15-23(34)16-25(35)36)32(21-11-9-20(29)10-12-21)30-27(26)28(37)31(3)17-19-7-5-4-6-8-19/h4-12,18,22-23,33-34H,13-17H2,1-3H3,(H,35,36)/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18417

((3R,5S)-6-({3-[(cyclohexylmethyl)(methyl)carbamoyl...)Show SMILES CC(C)c1c(nn(c1OC[C@@H](O)C[C@@H](O)CC(O)=O)-c1ccc(F)cc1)C(=O)N(C)CC1CCCCC1 |r| Show InChI InChI=1S/C27H38FN3O6/c1-17(2)24-25(26(36)30(3)15-18-7-5-4-6-8-18)29-31(20-11-9-19(28)10-12-20)27(24)37-16-22(33)13-21(32)14-23(34)35/h9-12,17-18,21-22,32-33H,4-8,13-16H2,1-3H3,(H,34,35)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215701

(CHEMBL250088 | sodium (3R,5R)-7-(3,4-bis(4-fluorop...)Show SMILES COc1cccc(NC(=O)c2c(c(c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)n2C(C)C)-c2ccc(F)cc2)-c2ccc(F)cc2)c1 Show InChI InChI=1S/C34H36F2N2O6/c1-20(2)38-29(16-15-26(39)18-27(40)19-30(41)42)31(21-7-11-23(35)12-8-21)32(22-9-13-24(36)14-10-22)33(38)34(43)37-25-5-4-6-28(17-25)44-3/h4-14,17,20,26-27,39-40H,15-16,18-19H2,1-3H3,(H,37,43)(H,41,42)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18399
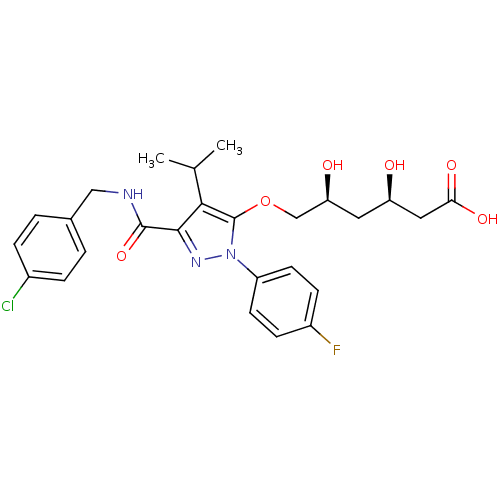
((3R,5S)-6-[(3-{[(4-chlorophenyl)methyl]carbamoyl}-...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1ccc(Cl)cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H29ClFN3O6/c1-15(2)23-24(25(36)29-13-16-3-5-17(27)6-4-16)30-31(19-9-7-18(28)8-10-19)26(23)37-14-21(33)11-20(32)12-22(34)35/h3-10,15,20-21,32-33H,11-14H2,1-2H3,(H,29,36)(H,34,35)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18405

((3R,5S)-6-[(3-{[(3-chlorophenyl)methyl](methyl)car...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)N(C)Cc1cccc(Cl)c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H31ClFN3O6/c1-16(2)24-25(26(37)31(3)14-17-5-4-6-18(28)11-17)30-32(20-9-7-19(29)8-10-20)27(24)38-15-22(34)12-21(33)13-23(35)36/h4-11,16,21-22,33-34H,12-15H2,1-3H3,(H,35,36)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18410

((3R,5S)-6-{[1-(4-fluorophenyl)-3-{[(2-fluorophenyl...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)N(C)Cc1ccccc1F)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H31F2N3O6/c1-16(2)24-25(26(37)31(3)14-17-6-4-5-7-22(17)29)30-32(19-10-8-18(28)9-11-19)27(24)38-15-21(34)12-20(33)13-23(35)36/h4-11,16,20-21,33-34H,12-15H2,1-3H3,(H,35,36)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215705

(CHEMBL399771 | sodium (3R,5R)-7-(3-(4-fluorophenyl...)Show SMILES CNC(=O)c1c(c(c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)n1C(C)C)-c1ccc(F)cc1)-c1ccccc1 Show InChI InChI=1S/C28H33FN2O5/c1-17(2)31-23(14-13-21(32)15-22(33)16-24(34)35)25(19-9-11-20(29)12-10-19)26(27(31)28(36)30-3)18-7-5-4-6-8-18/h4-12,17,21-22,32-33H,13-16H2,1-3H3,(H,30,36)(H,34,35)/p-1/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18401
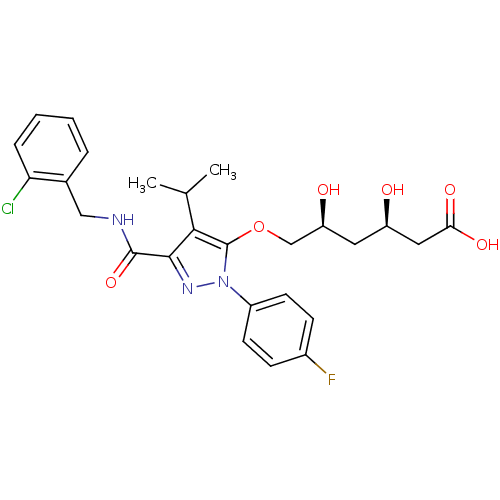
((3R,5S)-6-[(3-{[(2-chlorophenyl)methyl]carbamoyl}-...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1ccccc1Cl)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H29ClFN3O6/c1-15(2)23-24(25(36)29-13-16-5-3-4-6-21(16)27)30-31(18-9-7-17(28)8-10-18)26(23)37-14-20(33)11-19(32)12-22(34)35/h3-10,15,19-20,32-33H,11-14H2,1-2H3,(H,29,36)(H,34,35)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data