

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase (Candida glabrata) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Candida glabrata carbonic anhydrase by stopped flow CO2 hydration assay | Bioorg Med Chem Lett 19: 4802-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.048 BindingDB Entry DOI: 10.7270/Q2R212BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428619 (CHEMBL2331755) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428618 (CHEMBL2331756) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428621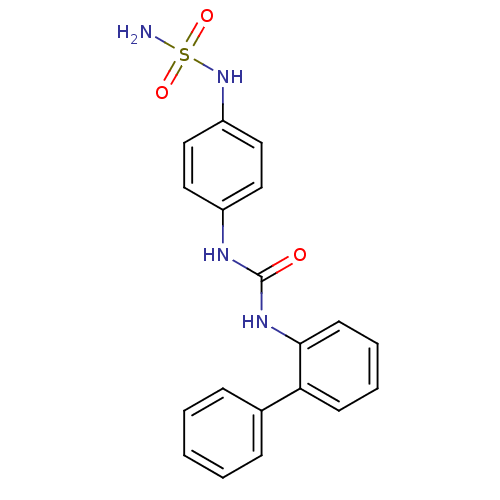 (CHEMBL2331768) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50433076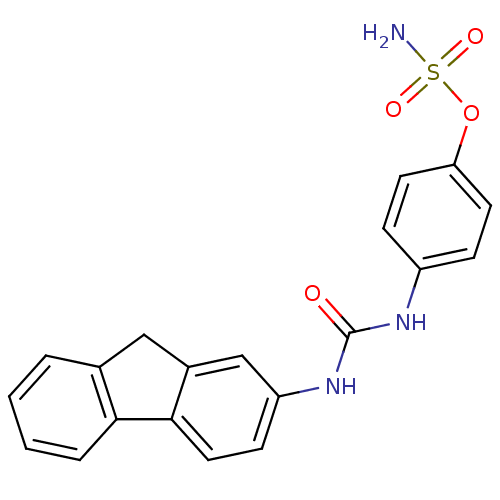 (CHEMBL2376275) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50433075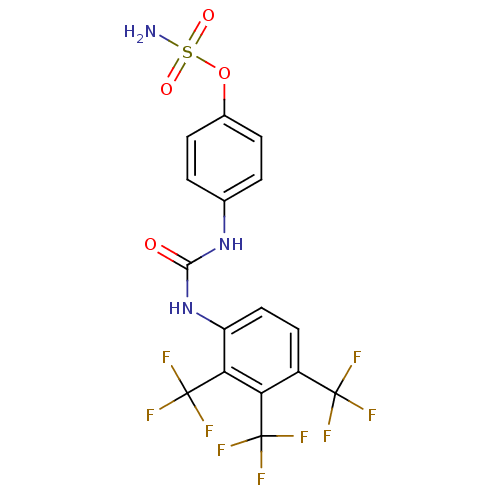 (CHEMBL2376276) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50387134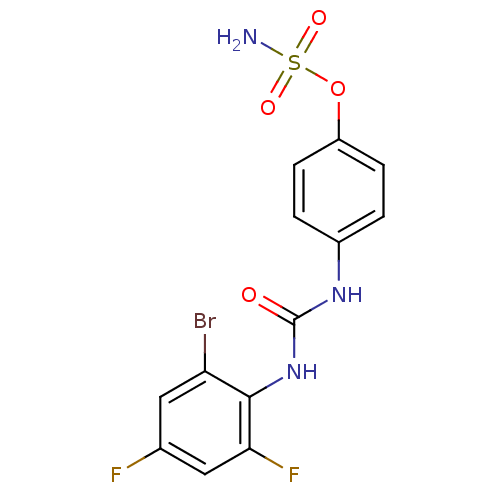 (4-ureidophenyl sulfamate ring derivative 3s | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428625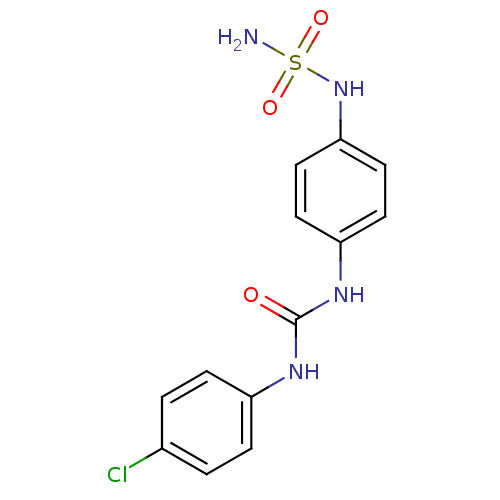 (CHEMBL2331764) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50387128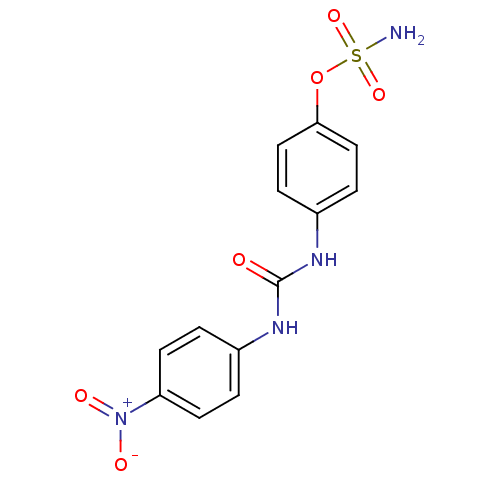 (4-ureidophenyl sulfamate ring derivative 3m | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428626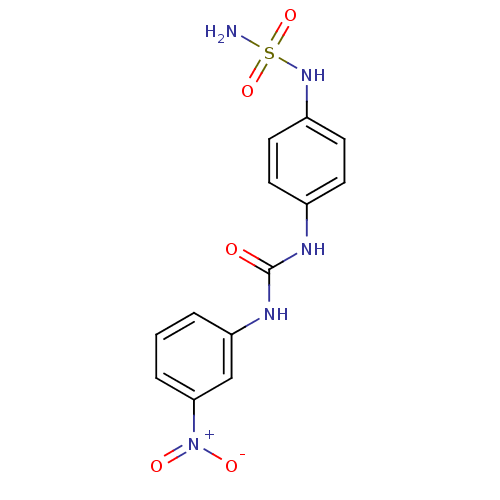 (CHEMBL2331763) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50433074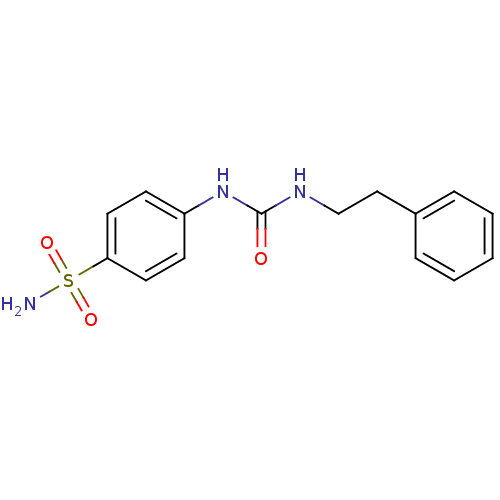 (CHEMBL2376273) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50387138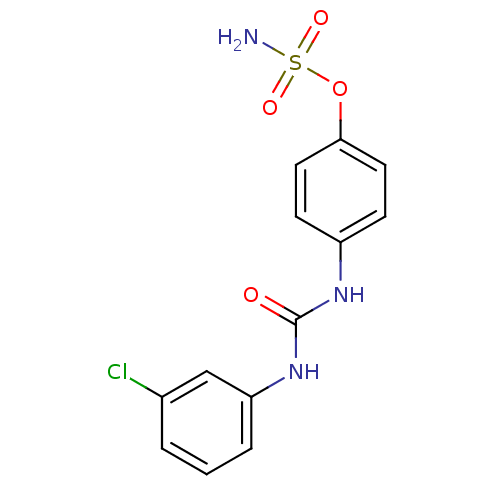 (CHEMBL2047818) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428624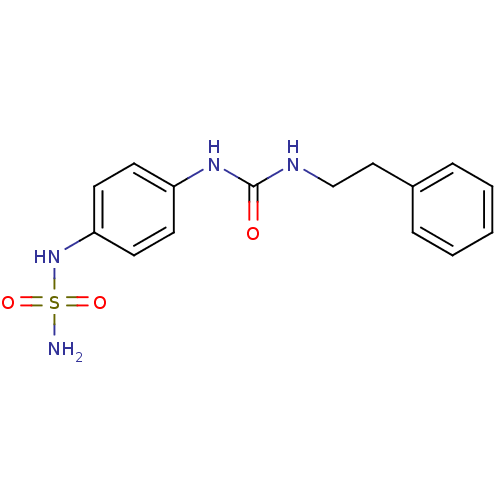 (CHEMBL2331765) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50387127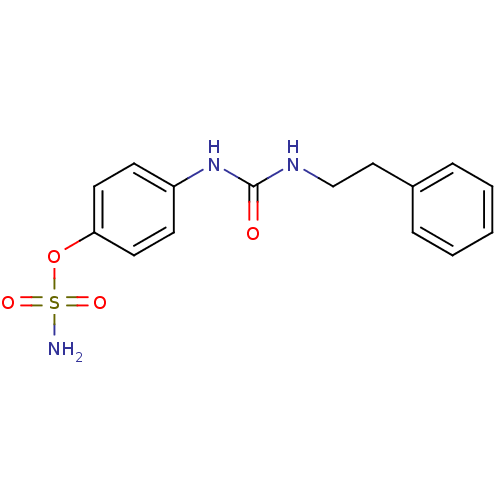 (4-ureidophenyl sulfamate ring derivative 3l | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428615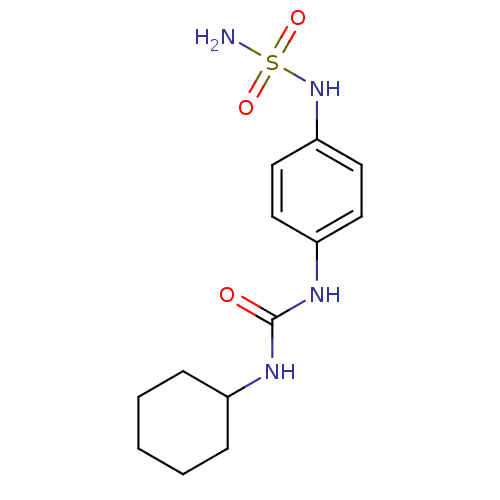 (CHEMBL2331759) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428627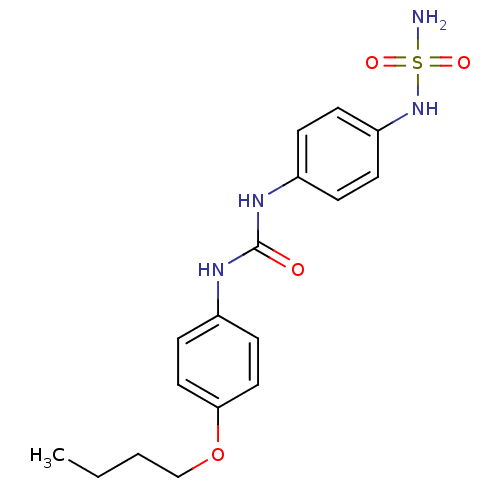 (CHEMBL2331762) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50433073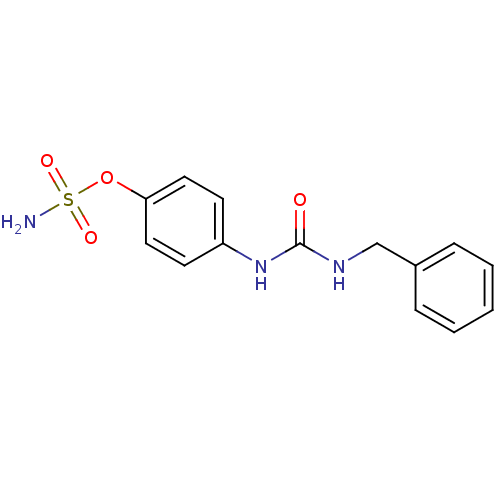 (CHEMBL2376274) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428623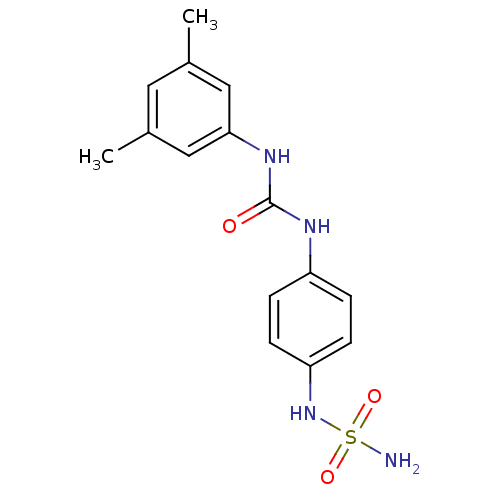 (CHEMBL2331766) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50387114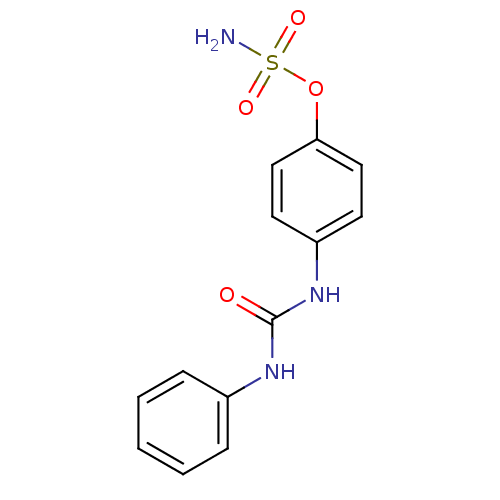 (CHEMBL2047796) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50428628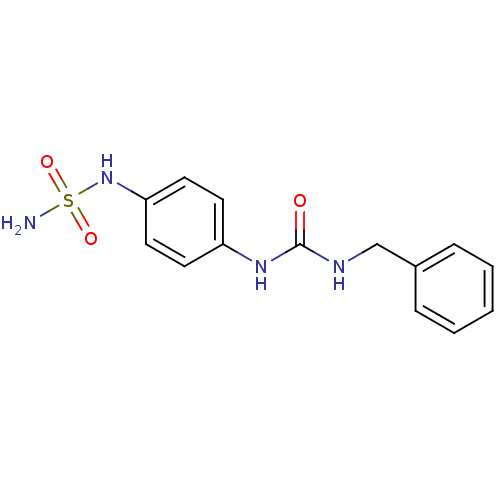 (CHEMBL2331761) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50387158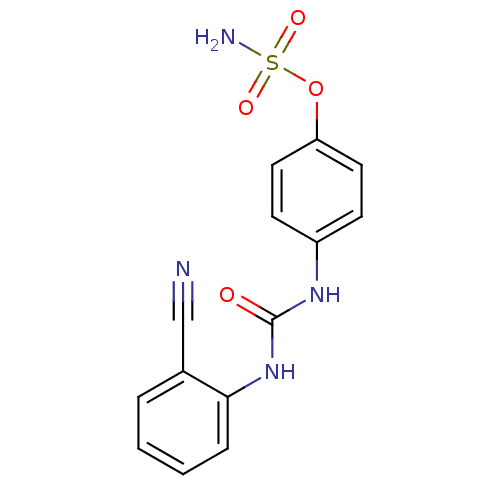 (CHEMBL2047839) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50387116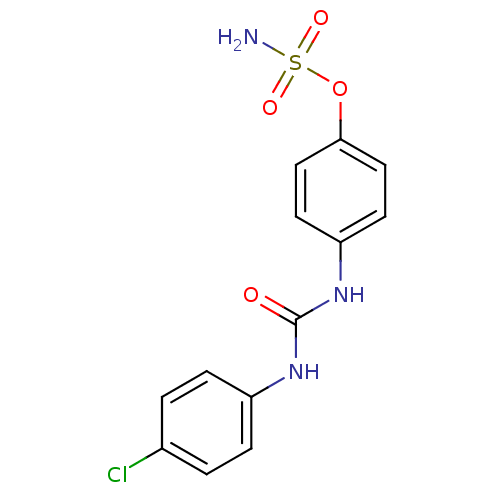 (4-ureidophenyl sulfamate ring derivative 3x | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida albicans (Yeast)) | BDBM50387133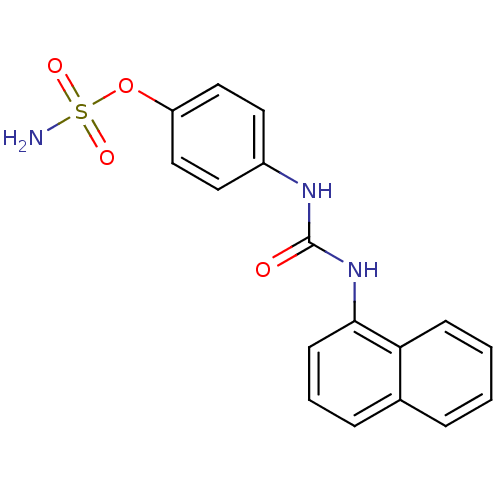 (CHEMBL2047813) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant full length Candida albicans NCE103 expressed in Escherichia coli BL21 preincubated for 15 mins by stopped-flow CO2 hydrase... | Bioorg Med Chem Lett 23: 2647-52 (2013) Article DOI: 10.1016/j.bmcl.2013.02.092 BindingDB Entry DOI: 10.7270/Q28S4R9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida glabrata) | BDBM26997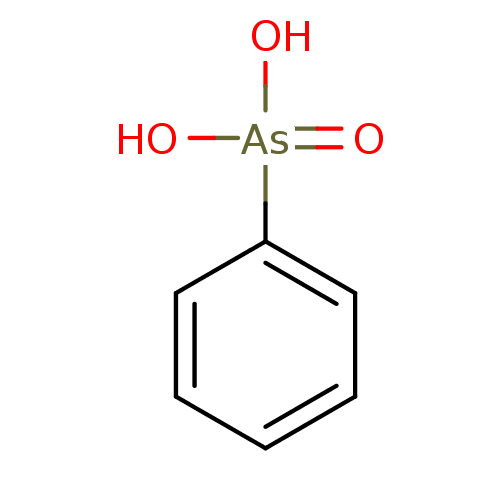 (CHEMBL364571 | PhAsO3H2 | benzenarsonic acid | ben...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Candida glabrata carbonic anhydrase by stopped flow CO2 hydration assay | Bioorg Med Chem Lett 19: 4802-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.048 BindingDB Entry DOI: 10.7270/Q2R212BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida glabrata) | BDBM26996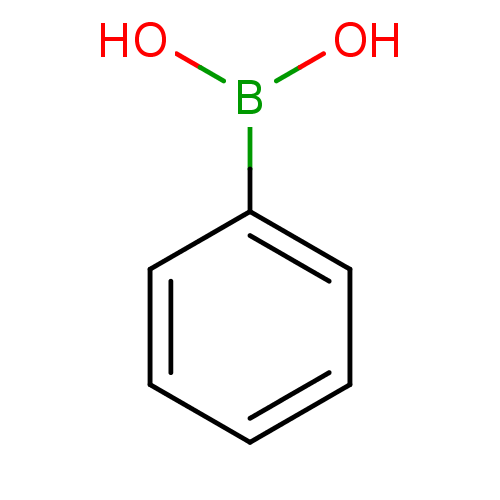 (CHEMBL21485 | PhB(OH)2 | Phenyl-boronic acid | Phe...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Candida glabrata carbonic anhydrase by stopped flow CO2 hydration assay | Bioorg Med Chem Lett 19: 4802-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.048 BindingDB Entry DOI: 10.7270/Q2R212BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida glabrata) | BDBM50297924 (CHEMBL232233 | H2NSO3H | sodium sulfamate) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Candida glabrata carbonic anhydrase by stopped flow CO2 hydration assay | Bioorg Med Chem Lett 19: 4802-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.048 BindingDB Entry DOI: 10.7270/Q2R212BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Candida glabrata) | BDBM26995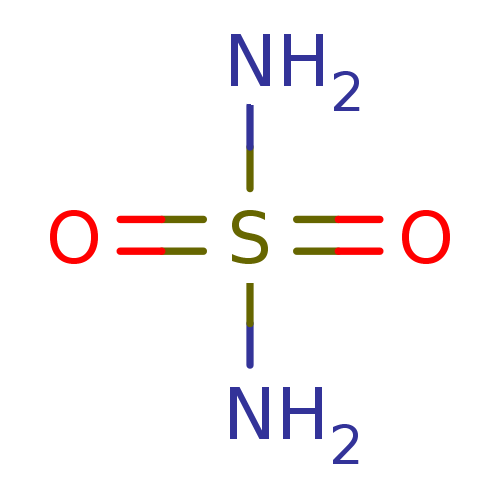 (CHEMBL355001 | H2NSO2NH2 | sulfamamide | sulfamide...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Candida glabrata carbonic anhydrase by stopped flow CO2 hydration assay | Bioorg Med Chem Lett 19: 4802-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.048 BindingDB Entry DOI: 10.7270/Q2R212BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50024146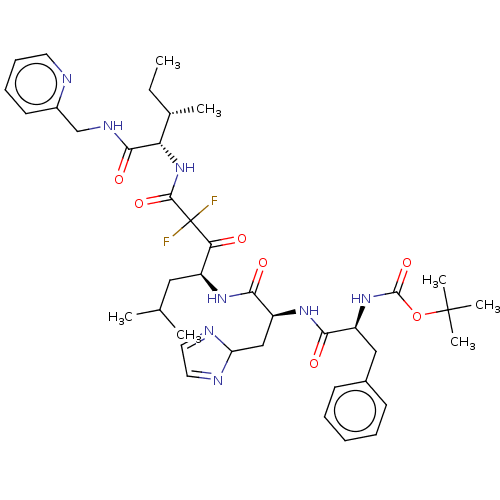 (CHEMBL3142169 | {1-[1-[1-(2,2-Difluoro-2-{2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 29: 2080-7 (1986) BindingDB Entry DOI: 10.7270/Q25X29G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50024149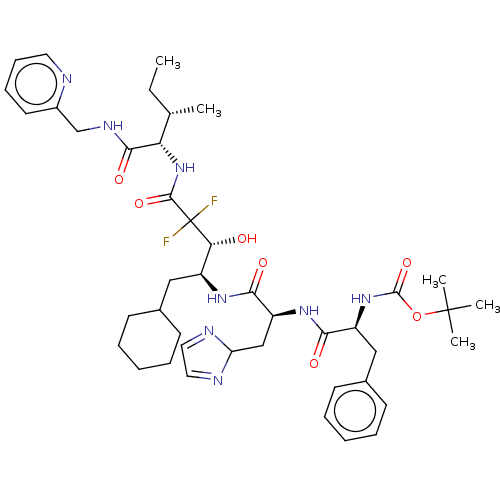 (CHEMBL3142178 | {1-[1-(1-Cyclohexylmethyl-3,3-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 29: 2080-7 (1986) BindingDB Entry DOI: 10.7270/Q25X29G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50024144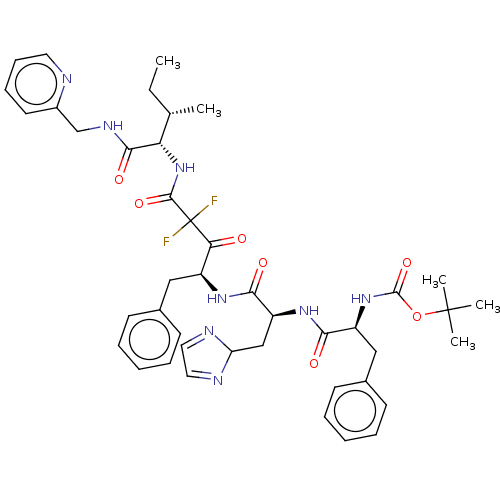 (CHEMBL3142193 | {1-[1-(1-Benzyl-3,3-difluoro-3-{2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 29: 2080-7 (1986) BindingDB Entry DOI: 10.7270/Q25X29G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50024145 (CHEMBL3350345 | {1-[1-(1-Cyclohexylmethyl-3,3-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 29: 2080-7 (1986) BindingDB Entry DOI: 10.7270/Q25X29G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021127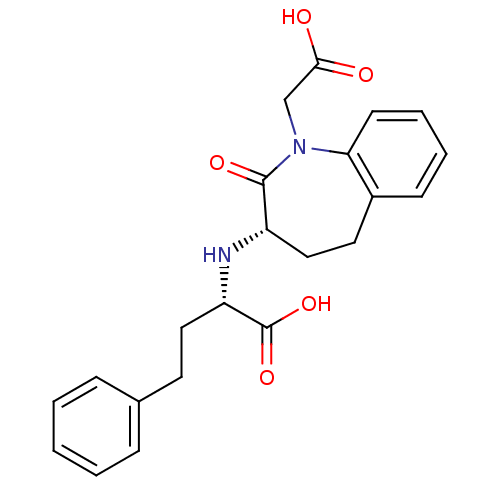 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1511-6 (1985) BindingDB Entry DOI: 10.7270/Q2BR8SRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021153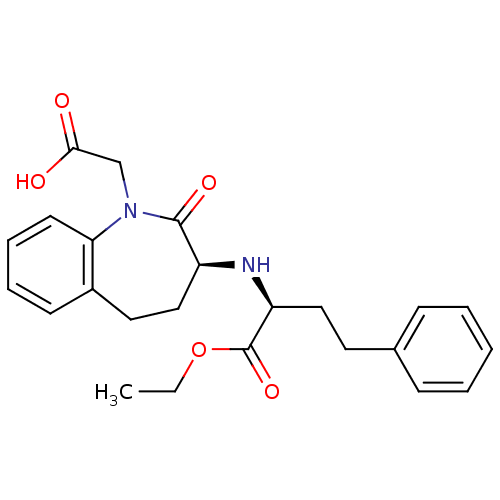 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 1603-6 (1985) BindingDB Entry DOI: 10.7270/Q2ZK5H7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021127 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1511-6 (1985) BindingDB Entry DOI: 10.7270/Q2BR8SRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50024147 (CHEMBL3142170 | {1-[1-[1-(1-Hydroxy-2-{2-methyl-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 29: 2080-7 (1986) BindingDB Entry DOI: 10.7270/Q25X29G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-2A adrenergic receptor (CALF-BOVINE) | BDBM50027226 (3-Methyl-1,2,3,4,4a,8-hexahydro-7-thia-3,12b-diaza...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards alpha-adrenoceptor of calf cortex membrane Site A using [3H]clonidine | J Med Chem 26: 1116-22 (1983) BindingDB Entry DOI: 10.7270/Q2SN07ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50024143 (CHEMBL3142184 | {1-[1-[1-(2,2-Difluoro-2-{2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 29: 2080-7 (1986) BindingDB Entry DOI: 10.7270/Q25X29G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021790 ((S,S)1-(3-Mercapto-2-methyl-propionyl)-2,3-dihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Angiotensin I converting enzyme was determined | J Med Chem 27: 816-8 (1984) BindingDB Entry DOI: 10.7270/Q20K2956 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021127 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1511-6 (1985) BindingDB Entry DOI: 10.7270/Q2BR8SRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50021148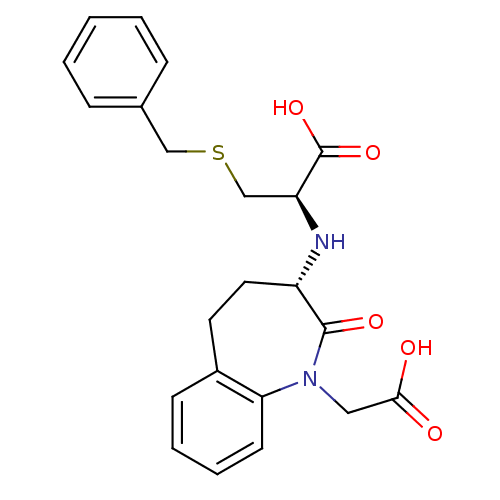 (3-Benzylsulfanyl-2-(1-carboxymethyl-2-oxo-2,3,4,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 1603-6 (1985) BindingDB Entry DOI: 10.7270/Q2ZK5H7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-2A adrenergic receptor (CALF-BOVINE) | BDBM50027226 (3-Methyl-1,2,3,4,4a,8-hexahydro-7-thia-3,12b-diaza...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards alpha-adrenoceptor of calf cortex membrane Site B using [3H]-clonidine | J Med Chem 26: 1116-22 (1983) BindingDB Entry DOI: 10.7270/Q2SN07ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-2A adrenergic receptor (CALF-BOVINE) | BDBM50027227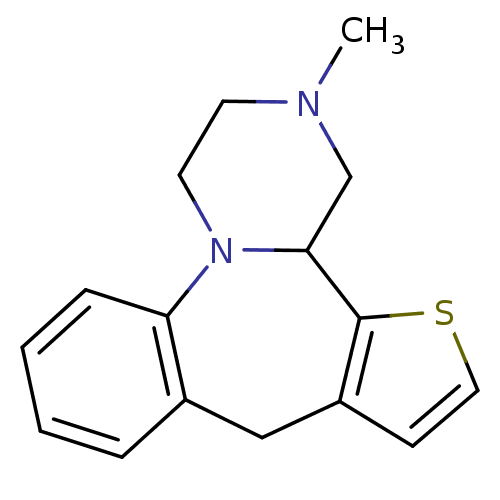 (3-Methyl-1,2,3,4,4a,8-hexahydro-5-thia-3,12b-diaza...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards alpha-adrenoceptor of calf cortex membrane Site B using [3H]-clonidine | J Med Chem 26: 1116-22 (1983) BindingDB Entry DOI: 10.7270/Q2SN07ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-2A adrenergic receptor (CALF-BOVINE) | BDBM50027227 (3-Methyl-1,2,3,4,4a,8-hexahydro-5-thia-3,12b-diaza...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards alpha-adrenoceptor of calf cortex membrane Site A using [3H]clonidine | J Med Chem 26: 1116-22 (1983) BindingDB Entry DOI: 10.7270/Q2SN07ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50021151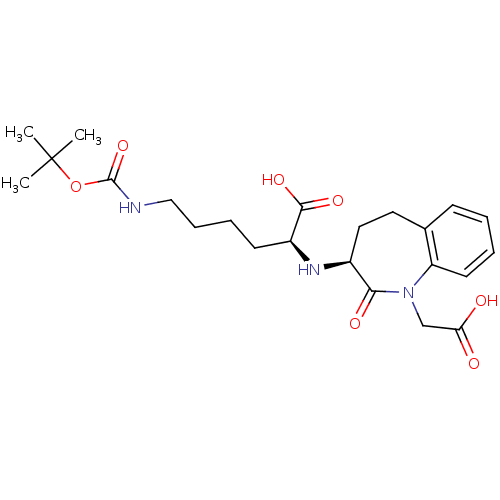 (6-tert-Butoxycarbonylamino-2-(1-carboxymethyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 1603-6 (1985) BindingDB Entry DOI: 10.7270/Q2ZK5H7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50027841 ((3-Mercaptomethyl-2-oxo-3,4,5,6-tetrahydro-2H-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Angiotensin I converting enzyme was determined | J Med Chem 27: 816-8 (1984) BindingDB Entry DOI: 10.7270/Q20K2956 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50021138 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 1603-6 (1985) BindingDB Entry DOI: 10.7270/Q2ZK5H7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50017129 ((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 1603-6 (1985) BindingDB Entry DOI: 10.7270/Q2ZK5H7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021129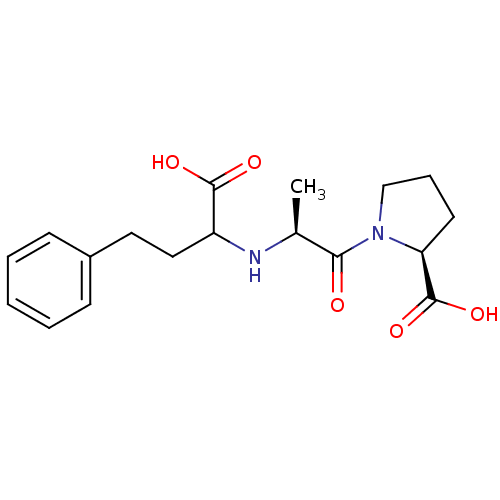 (1-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1511-6 (1985) BindingDB Entry DOI: 10.7270/Q2BR8SRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50021154 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 1603-6 (1985) BindingDB Entry DOI: 10.7270/Q2ZK5H7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021127 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1511-6 (1985) BindingDB Entry DOI: 10.7270/Q2BR8SRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 118 total ) | Next | Last >> |