

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase WNK1 (Homo sapiens (Human)) | BDBM50258546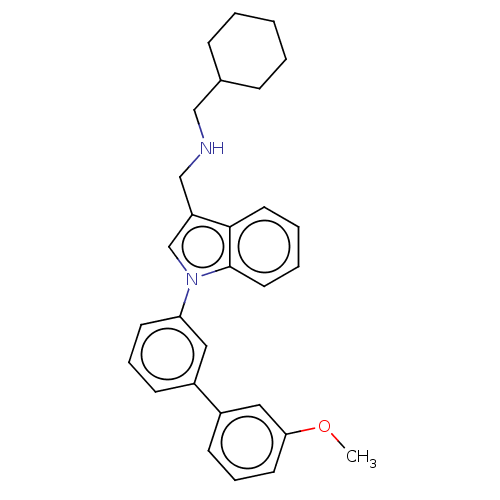 (CHEMBL4087727) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States. Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant human N-terminal GST-tagged WNK1 (1 to 491 residues) expressed in baculovirus expression system using fluor... | J Med Chem 60: 7099-7107 (2017) Article DOI: 10.1021/acs.jmedchem.7b00708 BindingDB Entry DOI: 10.7270/Q29W0HXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [166-489] (Homo sapiens (Human)) | BDBM203827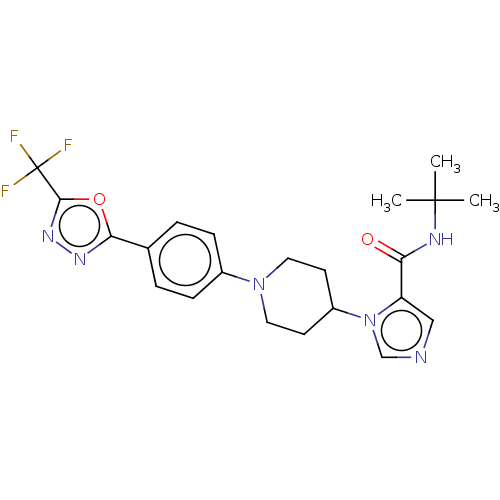 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 (Homo sapiens (Human)) | BDBM50258566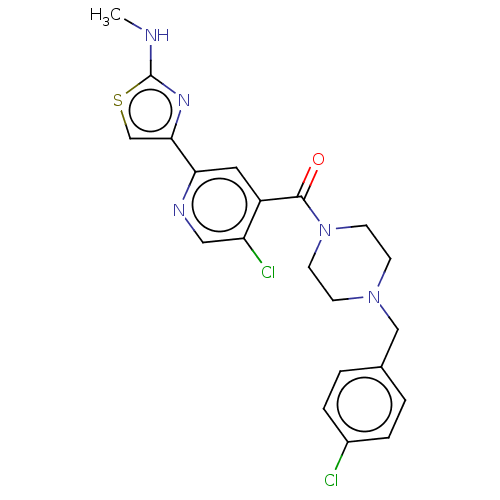 (CHEMBL4088706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States. Curated by ChEMBL | Assay Description Allosteric inhibition of recombinant human N-terminal GST-tagged WNK1 catalytic domain (1 to 491 residues) expressed in baculovirus expression system... | J Med Chem 60: 7099-7107 (2017) Article DOI: 10.1021/acs.jmedchem.7b00708 BindingDB Entry DOI: 10.7270/Q29W0HXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [1-491] (Homo sapiens (Human)) | BDBM203827 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 (Homo sapiens (Human)) | BDBM50258547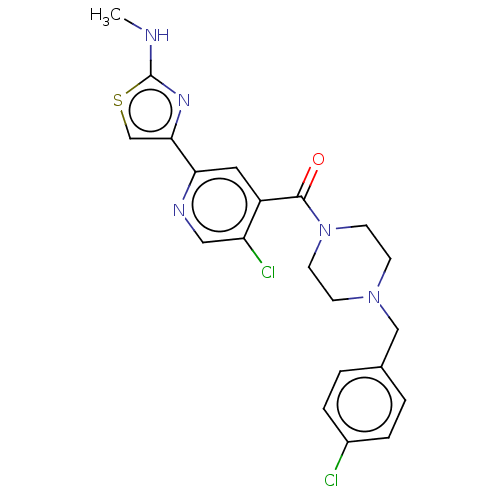 (CHEMBL4098876) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States. Curated by ChEMBL | Assay Description Allosteric inhibition of WNK1 (unknown origin) expressed in HEK293 cells co-expressing flag-OSR1 assessed as reduction in sorbitol-stimulated OSR1 ph... | J Med Chem 60: 7099-7107 (2017) Article DOI: 10.1021/acs.jmedchem.7b00708 BindingDB Entry DOI: 10.7270/Q29W0HXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [1-434] (Homo sapiens (Human)) | BDBM203827 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [1-444] (Homo sapiens (Human)) | BDBM203827 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066924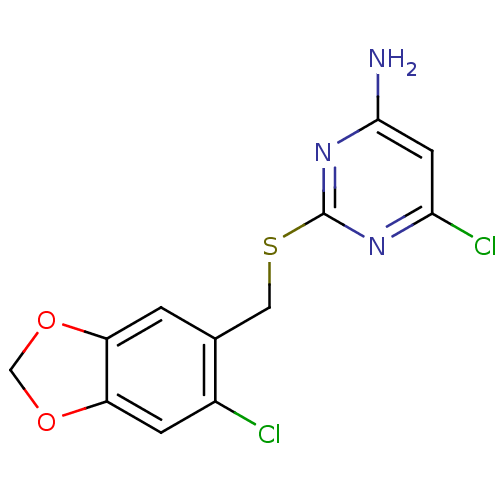 (6-Chloro-2-(6-chloro-benzo[1,3]dioxol-5-ylmethylsu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against E233V mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against wild type HIV-1 reverse transcriptase (WT-RT) | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009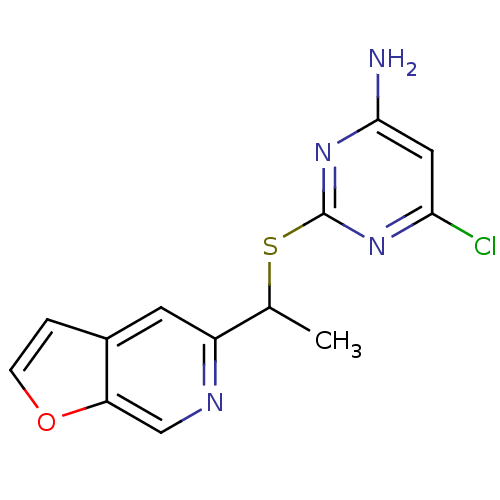 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against P236L mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against P236L mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against wild type HIV-1 reverse transcriptase (WT-RT) | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064008 (6-Chloro-2-((R)-1-furo[2,3-c]pyridin-5-yl-ethylsul...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against P236L mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against L100I mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase WNK1 (Homo sapiens (Human)) | BDBM50258598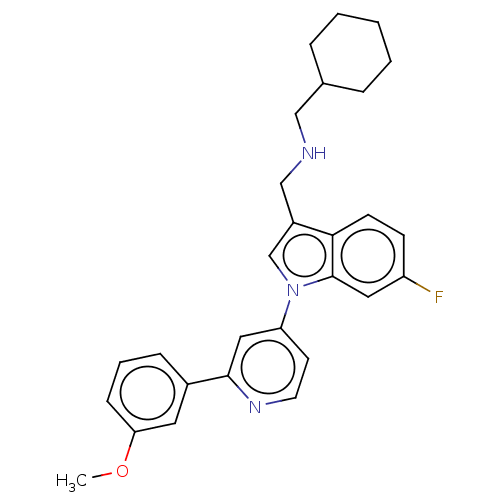 (CHEMBL4091128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States. Curated by ChEMBL | Assay Description Allosteric inhibition of WNK1 (unknown origin) expressed in HEK293 cells co-expressing flag-OSR1 assessed as reduction in sorbitol-stimulated OSR1 ph... | J Med Chem 60: 7099-7107 (2017) Article DOI: 10.1021/acs.jmedchem.7b00708 BindingDB Entry DOI: 10.7270/Q29W0HXP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase WNK1 [1-491] (Homo sapiens (Human)) | BDBM203827 (N-(tert-butyl)-1-(1-(5-(5-(trifluoromethyl)-1,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.3 | 4 |
Novartis Institutes | Assay Description Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ... | Nat Chem Biol 12: 896-898 (2016) Article DOI: 10.1038/nchembio.2168 BindingDB Entry DOI: 10.7270/Q2ZK5FH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against E233V mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against L100I mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066931 ((E)-4-(4-Amino-6-chloro-pyrimidin-2-ylsulfanyl)-bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066930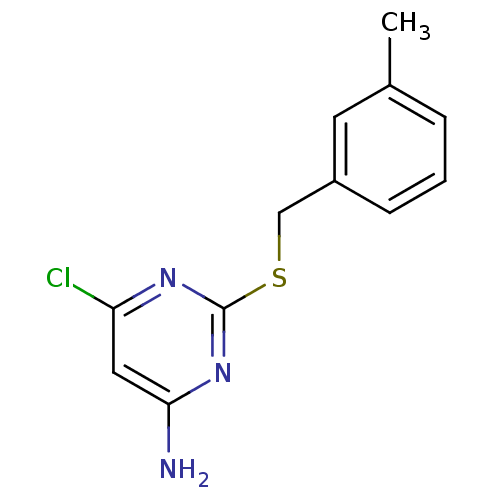 (6-Chloro-2-(3-methyl-benzylsulfanyl)-pyrimidin-4-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK2 [166-489] (Homo sapiens (Human)) | BDBM207990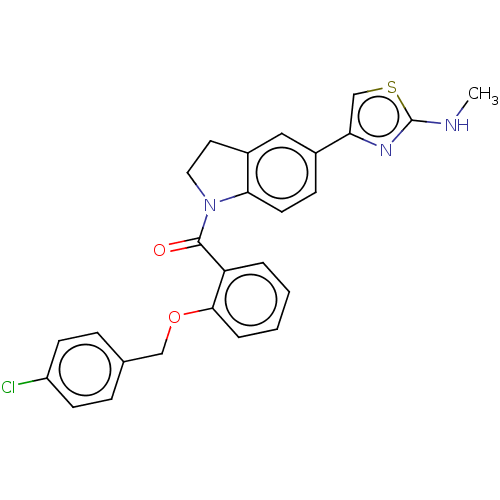 ((2-((4-Chlorobenzyl)oxy)phenyl)(5-(2-(methylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.3 | n/a |
Novartis Institutes for BioMedical Research, Inc. | Assay Description The assay utilized 5 to 10 nM of WNK1−4 protein compared to 25 nM used for mobility shift assay, enabling a more accurate comparison of selecti... | ACS Chem Biol 11: 3338-3346 (2016) Article DOI: 10.1021/acschembio.6b00511 BindingDB Entry DOI: 10.7270/Q2FB51SQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against Y188H mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase WNK1 (Homo sapiens (Human)) | BDBM50258595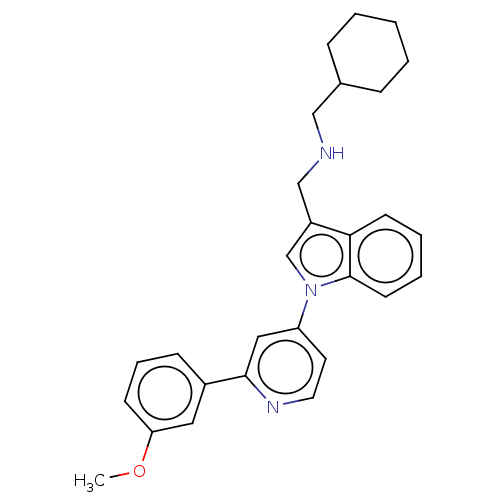 (CHEMBL4065531) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States. Curated by ChEMBL | Assay Description Allosteric inhibition of WNK1 (unknown origin) expressed in HEK293 cells co-expressing flag-OSR1 assessed as reduction in sorbitol-stimulated OSR1 ph... | J Med Chem 60: 7099-7107 (2017) Article DOI: 10.1021/acs.jmedchem.7b00708 BindingDB Entry DOI: 10.7270/Q29W0HXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066931 ((E)-4-(4-Amino-6-chloro-pyrimidin-2-ylsulfanyl)-bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (wild type) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50081628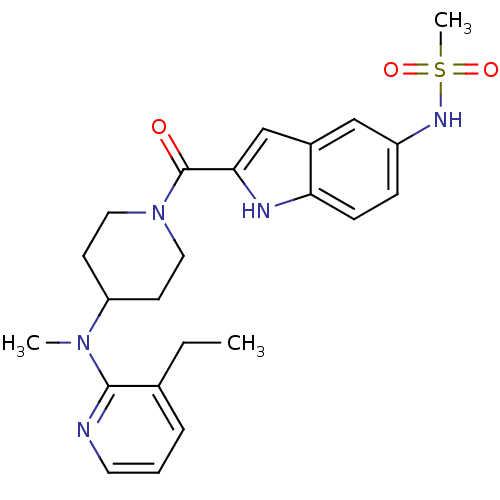 (CHEMBL336635 | N-(2-{4-[(3-Ethyl-pyridin-2-yl)-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of Reverse transcriptase (Wild Type) with poly(rA)600:oligo(dT)10 template primer | J Med Chem 42: 4140-9 (1999) BindingDB Entry DOI: 10.7270/Q2000192 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066932 (6-Chloro-2-(3,4-dichloro-benzylsulfanyl)-pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [1-491] (Homo sapiens (Human)) | BDBM207990 ((2-((4-Chlorobenzyl)oxy)phenyl)(5-(2-(methylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | 7.3 | n/a |
Novartis Institutes for BioMedical Research, Inc. | Assay Description The assay utilized 5 to 10 nM of WNK1−4 protein compared to 25 nM used for mobility shift assay, enabling a more accurate comparison of selecti... | ACS Chem Biol 11: 3338-3346 (2016) Article DOI: 10.1021/acschembio.6b00511 BindingDB Entry DOI: 10.7270/Q2FB51SQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066934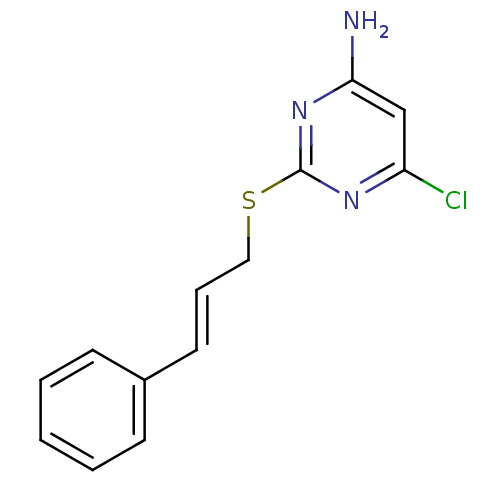 (6-Chloro-2-((E)-3-phenyl-allylsulfanyl)-pyrimidin-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066937 ((E)-4-(4-Amino-6-chloro-pyrimidin-2-ylsulfanyl)-bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against Y188H mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064008 (6-Chloro-2-((R)-1-furo[2,3-c]pyridin-5-yl-ethylsul...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against wild type HIV-1 reverse transcriptase (WT-RT) | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066924 (6-Chloro-2-(6-chloro-benzo[1,3]dioxol-5-ylmethylsu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description inhibitory activity against HIV-1 reverse transcriptase (wild type | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1954 ((Alkylamino)piperidine BHAP Analog 10 | 1-[(5-Meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 8.3 | 28 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 3769-89 (1996) Article DOI: 10.1021/jm960158n BindingDB Entry DOI: 10.7270/Q2XK8CQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 (Homo sapiens (Human)) | BDBM50258581 (CHEMBL4070177) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States. Curated by ChEMBL | Assay Description Allosteric inhibition of WNK1 (unknown origin) expressed in HEK293 cells co-expressing flag-OSR1 assessed as reduction in sorbitol-stimulated OSR1 ph... | J Med Chem 60: 7099-7107 (2017) Article DOI: 10.1021/acs.jmedchem.7b00708 BindingDB Entry DOI: 10.7270/Q29W0HXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50081631 (CHEMBL336807 | N-(2-{4-[Ethyl-(3-ethyl-pyridin-2-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of Reverse transcriptase (Wild Type) with poly(rA)600:oligo(dT)10 template primer | J Med Chem 42: 4140-9 (1999) BindingDB Entry DOI: 10.7270/Q2000192 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 (Homo sapiens (Human)) | BDBM50258567 (CHEMBL4060478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc. , Cambridge, Massachusetts 02139-4133, United States. Curated by ChEMBL | Assay Description Allosteric inhibition of WNK1 (unknown origin) expressed in HEK293 cells co-expressing flag-OSR1 assessed as reduction in sorbitol-stimulated OSR1 ph... | J Med Chem 60: 7099-7107 (2017) Article DOI: 10.1021/acs.jmedchem.7b00708 BindingDB Entry DOI: 10.7270/Q29W0HXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,P823L]/[588-1147,P823L] (Human immunodeficiency virus type 1) | BDBM1317 (3-[[(4,7-Dichlorobenzoxazol-2-yl)-methyl]amino]-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 3769-89 (1996) Article DOI: 10.1021/jm960158n BindingDB Entry DOI: 10.7270/Q2XK8CQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1966 ((Alkylamino)piperidine BHAP Analog 22 | 1-[(5-Meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 8.3 | 28 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 3769-89 (1996) Article DOI: 10.1021/jm960158n BindingDB Entry DOI: 10.7270/Q2XK8CQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50081622 (CHEMBL132770 | N-(2-{4-[(3-Ethyl-pyridin-2-yl)-pro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of Reverse transcriptase (Wild Type) with poly(rA)600:oligo(dT)10 template primer | J Med Chem 42: 4140-9 (1999) BindingDB Entry DOI: 10.7270/Q2000192 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50081624 (CHEMBL132491 | N-(2-{4-[(3-Methoxymethyl-pyridin-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of Reverse transcriptase (Wild Type) with poly(rA)600:oligo(dT)10 template primer | J Med Chem 42: 4140-9 (1999) BindingDB Entry DOI: 10.7270/Q2000192 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50081625 (CHEMBL134106 | N-(2-{4-[Ethyl-(3-methoxymethyl-pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of Reverse transcriptase (Wild Type) with poly(rA)600:oligo(dT)10 template primer | J Med Chem 42: 4140-9 (1999) BindingDB Entry DOI: 10.7270/Q2000192 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1967 ((Alkylamino)piperidine BHAP Analog 23 | 1-[[5-[[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 8.3 | 28 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 3769-89 (1996) Article DOI: 10.1021/jm960158n BindingDB Entry DOI: 10.7270/Q2XK8CQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1969 ((Alkylamino)piperidine BHAP Analog 25 | 1-[[5-[[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 8.3 | 28 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 3769-89 (1996) Article DOI: 10.1021/jm960158n BindingDB Entry DOI: 10.7270/Q2XK8CQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066936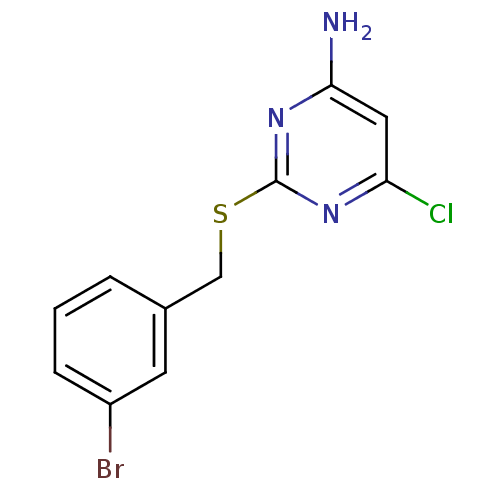 (2-(3-Bromo-benzylsulfanyl)-6-chloro-pyrimidin-4-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1964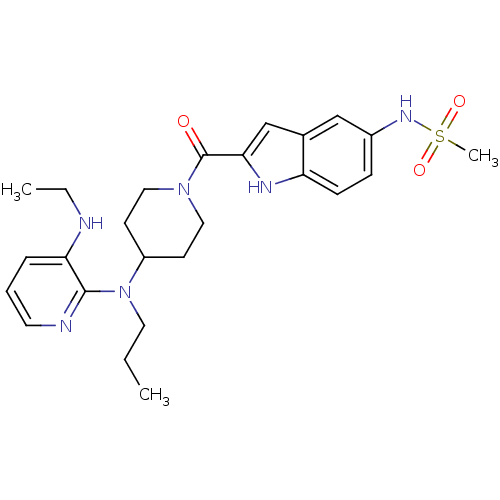 ((Alkylamino)piperidine BHAP Analog 20 | N-{2-[(4-{...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 8.3 | 28 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 3769-89 (1996) Article DOI: 10.1021/jm960158n BindingDB Entry DOI: 10.7270/Q2XK8CQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase WNK1 [1-491] (Homo sapiens (Human)) | BDBM207990 ((2-((4-Chlorobenzyl)oxy)phenyl)(5-(2-(methylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis Institutes for BioMedical Research, Inc. | Assay Description A mixture of fluorescein labeled OSR1 peptide substrate (Toray Research Center, Inc.) and ATP was prepared with final concentrations of 10 and 25 ... | ACS Chem Biol 11: 3338-3346 (2016) Article DOI: 10.1021/acschembio.6b00511 BindingDB Entry DOI: 10.7270/Q2FB51SQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066927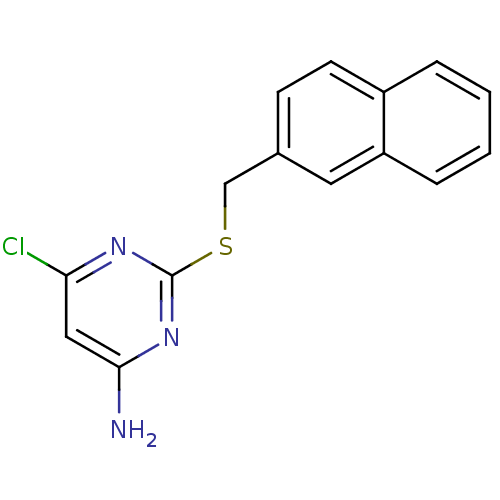 (6-Chloro-2-(naphthalen-2-ylmethylsulfanyl)-pyrimid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066925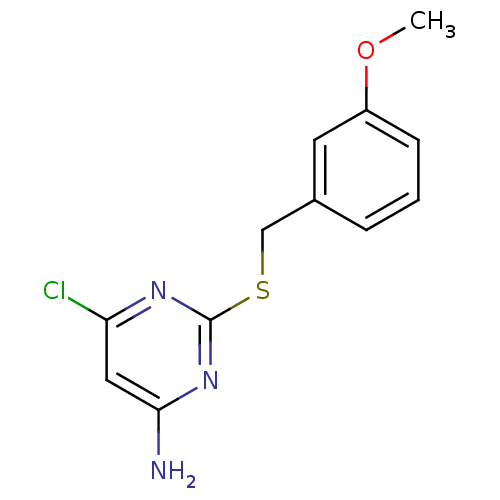 (6-Chloro-2-(3-methoxy-benzylsulfanyl)-pyrimidin-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1955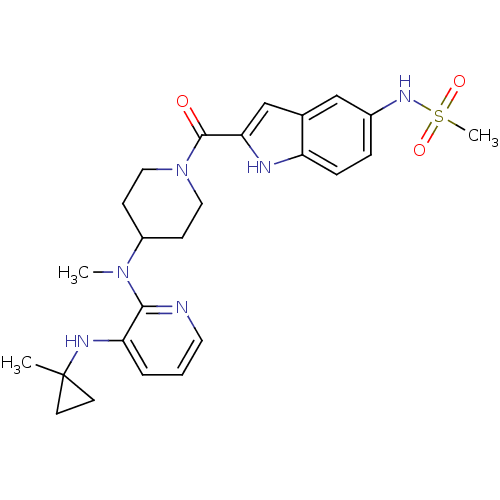 ((Alkylamino)piperidine BHAP Analog 11 | 1-[(5-Meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 8.3 | 28 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 3769-89 (1996) Article DOI: 10.1021/jm960158n BindingDB Entry DOI: 10.7270/Q2XK8CQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 321 total ) | Next | Last >> |