| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Coagulation factor XII |
|---|
| Ligand | BDBM50541586 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1984896 (CHEMBL4618302) |
|---|
| Ki | 1300±n/a nM |
|---|
| Citation |  Yang, W; Wang, Y; Lai, A; Clark, CG; Corte, JR; Fang, T; Gilligan, PJ; Jeon, Y; Pabbisetty, KB; Rampulla, RA; Mathur, A; Kaspady, M; Neithnadka, PR; Arumugam, A; Raju, S; Rossi, KA; Myers, JE; Sheriff, S; Lou, Z; Zheng, JJ; Chacko, SA; Bozarth, JM; Wu, Y; Crain, EJ; Wong, PC; Seiffert, DA; Luettgen, JM; Lam, PYS; Wexler, RR; Ewing, WR Discovery of a High Affinity, Orally Bioavailable Macrocyclic FXIa Inhibitor with Antithrombotic Activity in Preclinical Species. J Med Chem63:7226-7242 (2020) [PubMed] Article Yang, W; Wang, Y; Lai, A; Clark, CG; Corte, JR; Fang, T; Gilligan, PJ; Jeon, Y; Pabbisetty, KB; Rampulla, RA; Mathur, A; Kaspady, M; Neithnadka, PR; Arumugam, A; Raju, S; Rossi, KA; Myers, JE; Sheriff, S; Lou, Z; Zheng, JJ; Chacko, SA; Bozarth, JM; Wu, Y; Crain, EJ; Wong, PC; Seiffert, DA; Luettgen, JM; Lam, PYS; Wexler, RR; Ewing, WR Discovery of a High Affinity, Orally Bioavailable Macrocyclic FXIa Inhibitor with Antithrombotic Activity in Preclinical Species. J Med Chem63:7226-7242 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Coagulation factor XII |
|---|
| Name: | Coagulation factor XII |
|---|
| Synonyms: | Beta-factor XIIa part 1 | Beta-factor XIIa part 2 | Carboxylesterase 2 (intestine, liver) | Coagulation factor XII | Coagulation factor XII (FXII) | Coagulation factor XIIa heavy chain | Coagulation factor XIIa light chain | F12 | FA12_HUMAN | Factor XIIa | Factor XIIa (fXIIa) | HAF | Hageman factor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67810.07 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00748 |
|---|
| Residue: | 615 |
|---|
| Sequence: | MRALLLLGFLLVSLESTLSIPPWEAPKEHKYKAEEHTVVLTVTGEPCHFPFQYHRQLYHK
CTHKGRPGPQPWCATTPNFDQDQRWGYCLEPKKVKDHCSKHSPCQKGGTCVNMPSGPHCL
CPQHLTGNHCQKEKCFEPQLLRFFHKNEIWYRTEQAAVARCQCKGPDAHCQRLASQACRT
NPCLHGGRCLEVEGHRLCHCPVGYTGAFCDVDTKASCYDGRGLSYRGLARTTLSGAPCQP
WASEATYRNVTAEQARNWGLGGHAFCRNPDNDIRPWCFVLNRDRLSWEYCDLAQCQTPTQ
AAPPTPVSPRLHVPLMPAQPAPPKPQPTTRTPPQSQTPGALPAKREQPPSLTRNGPLSCG
QRLRKSLSSMTRVVGGLVALRGAHPYIAALYWGHSFCAGSLIAPCWVLTAAHCLQDRPAP
EDLTVVLGQERRNHSCEPCQTLAVRSYRLHEAFSPVSYQHDLALLRLQEDADGSCALLSP
YVQPVCLPSGAARPSETTLCQVAGWGHQFEGAEEYASFLQEAQVPFLSLERCSAPDVHGS
SILPGMLCAGFLEGGTDACQGDSGGPLVCEDQAAERRLTLQGIISWGSGCGDRNKPGVYT
DVAYYLAWIREHTVS
|
|
|
|---|
| BDBM50541586 |
|---|
| n/a |
|---|
| Name | BDBM50541586 |
|---|
| Synonyms: | CHEMBL4638245 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H29ClF2N4O4 |
|---|
| Mol. Mass. | 595.036 |
|---|
| SMILES | COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCC(=CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r,c:22| |
|---|
| Structure | 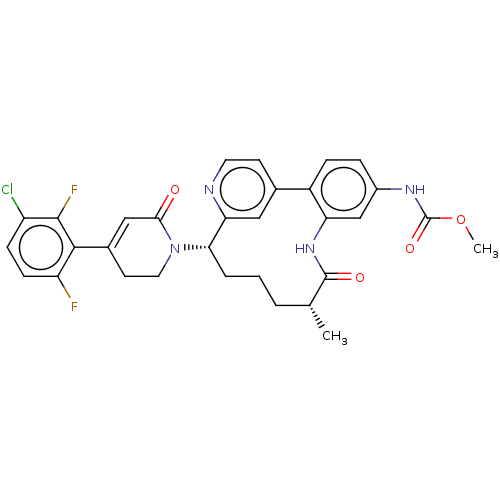
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yang, W; Wang, Y; Lai, A; Clark, CG; Corte, JR; Fang, T; Gilligan, PJ; Jeon, Y; Pabbisetty, KB; Rampulla, RA; Mathur, A; Kaspady, M; Neithnadka, PR; Arumugam, A; Raju, S; Rossi, KA; Myers, JE; Sheriff, S; Lou, Z; Zheng, JJ; Chacko, SA; Bozarth, JM; Wu, Y; Crain, EJ; Wong, PC; Seiffert, DA; Luettgen, JM; Lam, PYS; Wexler, RR; Ewing, WR Discovery of a High Affinity, Orally Bioavailable Macrocyclic FXIa Inhibitor with Antithrombotic Activity in Preclinical Species. J Med Chem63:7226-7242 (2020) [PubMed] Article
Yang, W; Wang, Y; Lai, A; Clark, CG; Corte, JR; Fang, T; Gilligan, PJ; Jeon, Y; Pabbisetty, KB; Rampulla, RA; Mathur, A; Kaspady, M; Neithnadka, PR; Arumugam, A; Raju, S; Rossi, KA; Myers, JE; Sheriff, S; Lou, Z; Zheng, JJ; Chacko, SA; Bozarth, JM; Wu, Y; Crain, EJ; Wong, PC; Seiffert, DA; Luettgen, JM; Lam, PYS; Wexler, RR; Ewing, WR Discovery of a High Affinity, Orally Bioavailable Macrocyclic FXIa Inhibitor with Antithrombotic Activity in Preclinical Species. J Med Chem63:7226-7242 (2020) [PubMed] Article