| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2A |
|---|
| Ligand | BDBM139370 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2222144 (CHEMBL5135478) |
|---|
| IC50 | 50±n/a nM |
|---|
| Citation |  Ma, M; Yang, Y; Du, G; Dai, Y; Zhu, X; Wang, W; Xu, H; Zhang, J; Zheng, L; Zou, F; Yang, H; Liu, B; Liu, W; Ye, L; Zhang, R; Tian, J Improving the treatment of Parkinson's disease: Structure-based development of novel 5-HT Eur J Med Chem234:0 (2022) [PubMed] Article Ma, M; Yang, Y; Du, G; Dai, Y; Zhu, X; Wang, W; Xu, H; Zhang, J; Zheng, L; Zou, F; Yang, H; Liu, B; Liu, W; Ye, L; Zhang, R; Tian, J Improving the treatment of Parkinson's disease: Structure-based development of novel 5-HT Eur J Med Chem234:0 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2A |
|---|
| Name: | 5-hydroxytryptamine receptor 2A |
|---|
| Synonyms: | 5-HT-2 | 5-HT-2A | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT-2A) | 5-hydroxytryptamine receptor 2A (5HT-2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_HUMAN | HTR2 | HTR2A | Serotonin receptor 2A |
|---|
| Type: | undefined |
|---|
| Mol. Mass.: | 52607.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P28223 |
|---|
| Residue: | 471 |
|---|
| Sequence: | MDILCEENTSLSSTTNSLMQLNDDTRLYSNDFNSGEANTSDAFNWTVDSENRTNLSCEGC
LSPSCLSLLHLQEKNWSALLTAVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIAD
MLLGFLVMPVSMLTILYGYRWPLPSKLCAVWIYLDVLFSTASIMHLCAISLDRYVAIQNP
IHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSF
VSFFIPLTIMVITYFLTIKSLQKEATLCVSDLGTRAKLASFSFLPQSSLSSEKLFQRSIH
REPGSYTGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNEDVIGA
LLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENKKPLQLILVNTIPALAYK
SSQLQMGQKKNSKQDAKTTDNDCSMVALGKQHSEEASKDNSDGVNEKVSCV
|
|
|
|---|
| BDBM139370 |
|---|
| n/a |
|---|
| Name | BDBM139370 |
|---|
| Synonyms: | ACP-103 | Nuplazid | Pimavanserin | Pimavanserin hydrochloride | Pimavanserin tartrate | US20230348421, Compound Pimavanserin | WO2023288027, Cmpd PIMA | bis(1-(4-fluorobenzyl)-1-(1-methylpiperidin-4-yl)-3-(4-(2-methylpropoxy)benzyl)urea) (2R,3R)-2,3-dihydroxybutanedioate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H34FN3O2 |
|---|
| Mol. Mass. | 427.5548 |
|---|
| SMILES | CC(C)COc1ccc(CNC(=O)N(Cc2ccc(F)cc2)C2CCN(C)CC2)cc1 |
|---|
| Structure | 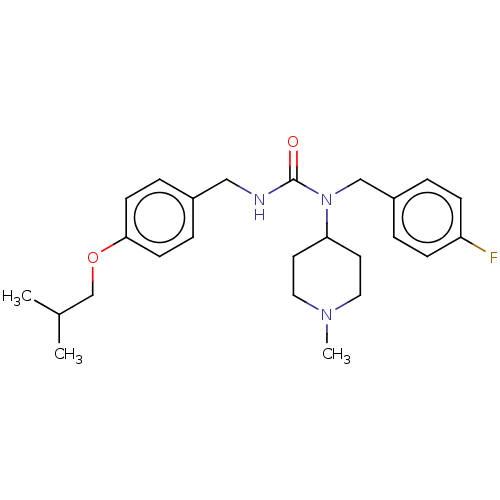
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ma, M; Yang, Y; Du, G; Dai, Y; Zhu, X; Wang, W; Xu, H; Zhang, J; Zheng, L; Zou, F; Yang, H; Liu, B; Liu, W; Ye, L; Zhang, R; Tian, J Improving the treatment of Parkinson's disease: Structure-based development of novel 5-HT Eur J Med Chem234:0 (2022) [PubMed] Article
Ma, M; Yang, Y; Du, G; Dai, Y; Zhu, X; Wang, W; Xu, H; Zhang, J; Zheng, L; Zou, F; Yang, H; Liu, B; Liu, W; Ye, L; Zhang, R; Tian, J Improving the treatment of Parkinson's disease: Structure-based development of novel 5-HT Eur J Med Chem234:0 (2022) [PubMed] Article