| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50186618 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_353252 (CHEMBL861848) |
|---|
| IC50 | 2000±n/a nM |
|---|
| Citation |  Morwick, T; Berry, A; Brickwood, J; Cardozo, M; Catron, K; DeTuri, M; Emeigh, J; Homon, C; Hrapchak, M; Jacober, S; Jakes, S; Kaplita, P; Kelly, TA; Ksiazek, J; Liuzzi, M; Magolda, R; Mao, C; Marshall, D; McNeil, D; Prokopowicz, A; Sarko, C; Scouten, E; Sledziona, C; Sun, S; Watrous, J; Wu, JP; Cywin, CL Evolution of the thienopyridine class of inhibitors of IkappaB kinase-beta: part I: hit-to-lead strategies. J Med Chem49:2898-908 (2006) [PubMed] Article Morwick, T; Berry, A; Brickwood, J; Cardozo, M; Catron, K; DeTuri, M; Emeigh, J; Homon, C; Hrapchak, M; Jacober, S; Jakes, S; Kaplita, P; Kelly, TA; Ksiazek, J; Liuzzi, M; Magolda, R; Mao, C; Marshall, D; McNeil, D; Prokopowicz, A; Sarko, C; Scouten, E; Sledziona, C; Sun, S; Watrous, J; Wu, JP; Cywin, CL Evolution of the thienopyridine class of inhibitors of IkappaB kinase-beta: part I: hit-to-lead strategies. J Med Chem49:2898-908 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50186618 |
|---|
| n/a |
|---|
| Name | BDBM50186618 |
|---|
| Synonyms: | 3-amino-5-(4-fluoro-phenyl)-thiophene-2-carboxylic acid amide | CHEMBL207071 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C11H9FN2OS |
|---|
| Mol. Mass. | 236.265 |
|---|
| SMILES | NC(=O)c1sc(cc1N)-c1ccc(F)cc1 |
|---|
| Structure | 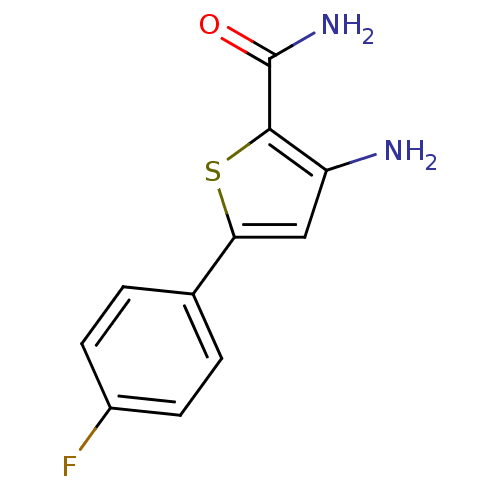
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Morwick, T; Berry, A; Brickwood, J; Cardozo, M; Catron, K; DeTuri, M; Emeigh, J; Homon, C; Hrapchak, M; Jacober, S; Jakes, S; Kaplita, P; Kelly, TA; Ksiazek, J; Liuzzi, M; Magolda, R; Mao, C; Marshall, D; McNeil, D; Prokopowicz, A; Sarko, C; Scouten, E; Sledziona, C; Sun, S; Watrous, J; Wu, JP; Cywin, CL Evolution of the thienopyridine class of inhibitors of IkappaB kinase-beta: part I: hit-to-lead strategies. J Med Chem49:2898-908 (2006) [PubMed] Article
Morwick, T; Berry, A; Brickwood, J; Cardozo, M; Catron, K; DeTuri, M; Emeigh, J; Homon, C; Hrapchak, M; Jacober, S; Jakes, S; Kaplita, P; Kelly, TA; Ksiazek, J; Liuzzi, M; Magolda, R; Mao, C; Marshall, D; McNeil, D; Prokopowicz, A; Sarko, C; Scouten, E; Sledziona, C; Sun, S; Watrous, J; Wu, JP; Cywin, CL Evolution of the thienopyridine class of inhibitors of IkappaB kinase-beta: part I: hit-to-lead strategies. J Med Chem49:2898-908 (2006) [PubMed] Article