| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Somatostatin receptor type 3 |
|---|
| Ligand | BDBM50091555 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1498161 (CHEMBL3583381) |
|---|
| IC50 | 11±n/a nM |
|---|
| Citation |  Shah, SK; He, S; Guo, L; Truong, Q; Qi, H; Du, W; Lai, Z; Liu, J; Jian, T; Hong, Q; Dobbelaar, P; Ye, Z; Sherer, E; Feng, Z; Yu, Y; Wong, F; Samuel, K; Madiera, M; Karanam, BV; Reddy, VB; Mitelman, S; Tong, SX; Chicchi, GG; Tsao, KL; Trusca, D; Feng, Y; Wu, M; Shao, Q; Trujillo, ME; Eiermann, GJ; Li, C; Pachanski, M; Fernandez, G; Nelson, D; Bunting, P; Morissette, P; Volksdorf, S; Kerr, J; Zhang, BB; Howard, AD; Zhou, YP; Pasternak, A; Nargund, RP; Hagmann, WK Discovery of MK-1421, a Potent, Selective sstr3 Antagonist, as a Development Candidate for Type 2 Diabetes. ACS Med Chem Lett6:513-7 (2015) [PubMed] Article Shah, SK; He, S; Guo, L; Truong, Q; Qi, H; Du, W; Lai, Z; Liu, J; Jian, T; Hong, Q; Dobbelaar, P; Ye, Z; Sherer, E; Feng, Z; Yu, Y; Wong, F; Samuel, K; Madiera, M; Karanam, BV; Reddy, VB; Mitelman, S; Tong, SX; Chicchi, GG; Tsao, KL; Trusca, D; Feng, Y; Wu, M; Shao, Q; Trujillo, ME; Eiermann, GJ; Li, C; Pachanski, M; Fernandez, G; Nelson, D; Bunting, P; Morissette, P; Volksdorf, S; Kerr, J; Zhang, BB; Howard, AD; Zhou, YP; Pasternak, A; Nargund, RP; Hagmann, WK Discovery of MK-1421, a Potent, Selective sstr3 Antagonist, as a Development Candidate for Type 2 Diabetes. ACS Med Chem Lett6:513-7 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Somatostatin receptor type 3 |
|---|
| Name: | Somatostatin receptor type 3 |
|---|
| Synonyms: | SOMATOSTATIN SST3 | SS-3-R | SS3-R | SS3R | SSR-28 | SSR3_HUMAN | SSTR3 | Somatostatin receptor type 3 (SSTR3) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 45855.97 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P32745 |
|---|
| Residue: | 418 |
|---|
| Sequence: | MDMLHPSSVSTTSEPENASSAWPPDATLGNVSAGPSPAGLAVSGVLIPLVYLVVCVVGLL
GNSLVIYVVLRHTASPSVTNVYILNLALADELFMLGLPFLAAQNALSYWPFGSLMCRLVM
AVDGINQFTSIFCLTVMSVDRYLAVVHPTRSARWRTAPVARTVSAAVWVASAVVVLPVVV
FSGVPRGMSTCHMQWPEPAAAWRAGFIIYTAALGFFGPLLVICLCYLLIVVKVRSAGRRV
WAPSCQRRRRSERRVTRMVVAVVALFVLCWMPFYVLNIVNVVCPLPEEPAFFGLYFLVVA
LPYANSCANPILYGFLSYRFKQGFRRVLLRPSRRVRSQEPTVGPPEKTEEEDEEEEDGEE
SREGGKGKEMNGRVSQITQPGTSGQERPPSRVASKEQQLLPQEASTGEKSSTMRISYL
|
|
|
|---|
| BDBM50091555 |
|---|
| n/a |
|---|
| Name | BDBM50091555 |
|---|
| Synonyms: | CHEMBL3582307 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H17FN4 |
|---|
| Mol. Mass. | 332.3742 |
|---|
| SMILES | Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c(CN1)[nH]c1ccccc21 |r| |
|---|
| Structure | 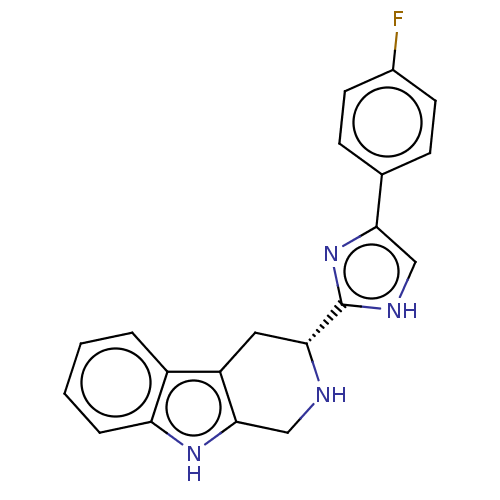
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Shah, SK; He, S; Guo, L; Truong, Q; Qi, H; Du, W; Lai, Z; Liu, J; Jian, T; Hong, Q; Dobbelaar, P; Ye, Z; Sherer, E; Feng, Z; Yu, Y; Wong, F; Samuel, K; Madiera, M; Karanam, BV; Reddy, VB; Mitelman, S; Tong, SX; Chicchi, GG; Tsao, KL; Trusca, D; Feng, Y; Wu, M; Shao, Q; Trujillo, ME; Eiermann, GJ; Li, C; Pachanski, M; Fernandez, G; Nelson, D; Bunting, P; Morissette, P; Volksdorf, S; Kerr, J; Zhang, BB; Howard, AD; Zhou, YP; Pasternak, A; Nargund, RP; Hagmann, WK Discovery of MK-1421, a Potent, Selective sstr3 Antagonist, as a Development Candidate for Type 2 Diabetes. ACS Med Chem Lett6:513-7 (2015) [PubMed] Article
Shah, SK; He, S; Guo, L; Truong, Q; Qi, H; Du, W; Lai, Z; Liu, J; Jian, T; Hong, Q; Dobbelaar, P; Ye, Z; Sherer, E; Feng, Z; Yu, Y; Wong, F; Samuel, K; Madiera, M; Karanam, BV; Reddy, VB; Mitelman, S; Tong, SX; Chicchi, GG; Tsao, KL; Trusca, D; Feng, Y; Wu, M; Shao, Q; Trujillo, ME; Eiermann, GJ; Li, C; Pachanski, M; Fernandez, G; Nelson, D; Bunting, P; Morissette, P; Volksdorf, S; Kerr, J; Zhang, BB; Howard, AD; Zhou, YP; Pasternak, A; Nargund, RP; Hagmann, WK Discovery of MK-1421, a Potent, Selective sstr3 Antagonist, as a Development Candidate for Type 2 Diabetes. ACS Med Chem Lett6:513-7 (2015) [PubMed] Article