 Found 170 hits with Last Name = 'madiera' and Initial = 'm'
Found 170 hits with Last Name = 'madiera' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50091554
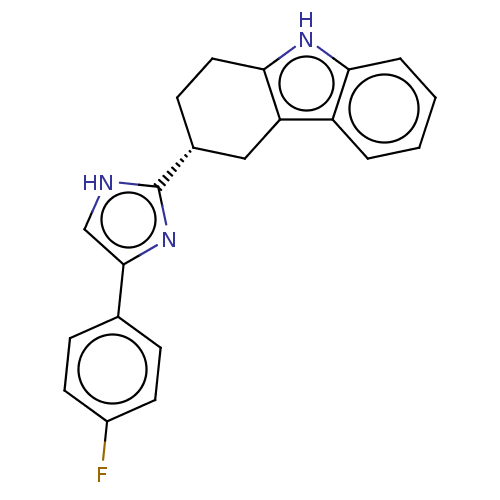
(CHEMBL3582308)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@@H]1CCc2[nH]c3ccccc3c2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50389592
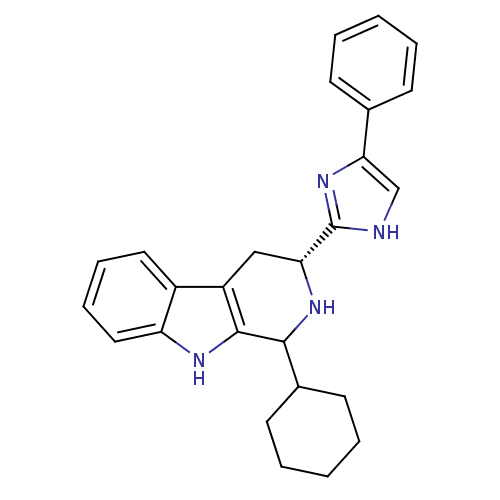
(CHEMBL2069500)Show SMILES C1CCC(CC1)C1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C26H28N4/c1-3-9-17(10-4-1)23-16-27-26(30-23)22-15-20-19-13-7-8-14-21(19)28-25(20)24(29-22)18-11-5-2-6-12-18/h1,3-4,7-10,13-14,16,18,22,24,28-29H,2,5-6,11-12,15H2,(H,27,30)/t22-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50091555
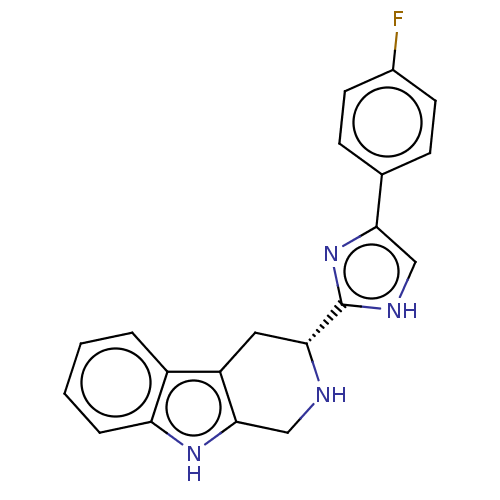
(CHEMBL3582307)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c(CN1)[nH]c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50091553
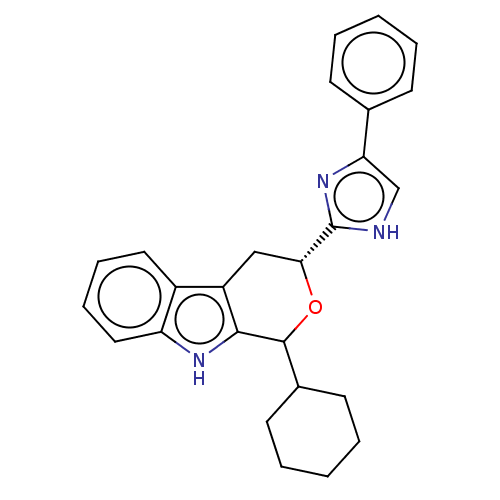
(CHEMBL3582309)Show SMILES C1CCC(CC1)C1O[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50091558

(CHEMBL3582311)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)C(N1)(c1ccccc1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50091549
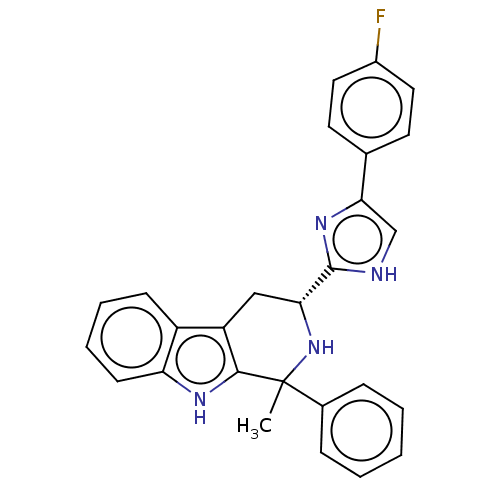
(CHEMBL3582310)Show SMILES CC1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1ccccc1 |r| Show InChI InChI=1S/C29H36O15/c1-13-22(36)23(37)24(38)29(41-13)44-27-25(39)28(40-9-8-15-3-6-17(32)19(34)11-15)42-20(12-30)26(27)43-21(35)7-4-14-2-5-16(31)18(33)10-14/h2-7,10-11,13,20,22-34,36-39H,8-9,12H2,1H3/b7-4+/t13?,20-,22?,23?,24?,25-,26-,27-,28-,29?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115402
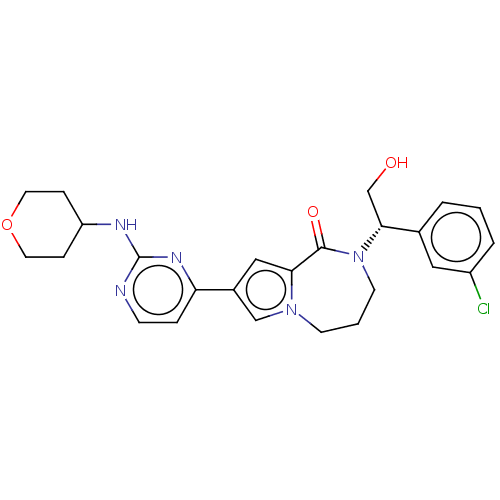
(CHEMBL3608588)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H28ClN5O3/c26-19-4-1-3-17(13-19)23(16-32)31-10-2-9-30-15-18(14-22(30)24(31)33)21-5-8-27-25(29-21)28-20-6-11-34-12-7-20/h1,3-5,8,13-15,20,23,32H,2,6-7,9-12,16H2,(H,27,28,29)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115401
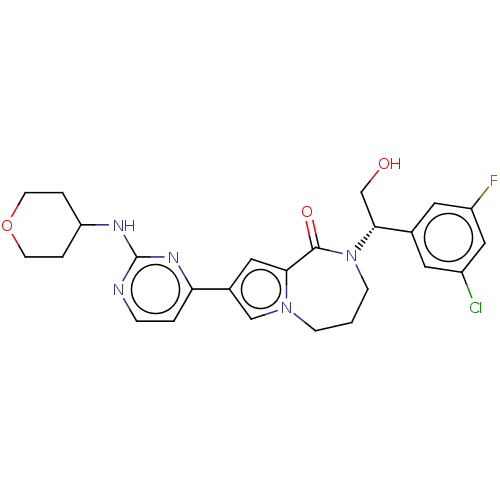
(CHEMBL3608589)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C25H27ClFN5O3/c26-18-10-16(11-19(27)13-18)23(15-33)32-7-1-6-31-14-17(12-22(31)24(32)34)21-2-5-28-25(30-21)29-20-3-8-35-9-4-20/h2,5,10-14,20,23,33H,1,3-4,6-9,15H2,(H,28,29,30)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115398
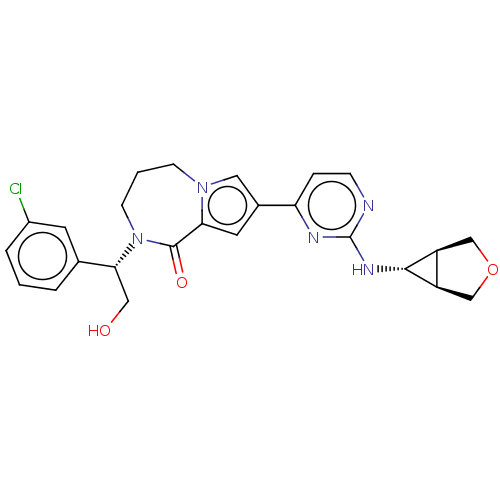
(CHEMBL3608586)Show SMILES [H][C@@]12COC[C@]1([H])[C@H]2Nc1nccc(n1)-c1cc2C(=O)N(CCCn2c1)[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26ClN5O3/c26-17-4-1-3-15(9-17)22(12-32)31-8-2-7-30-11-16(10-21(30)24(31)33)20-5-6-27-25(28-20)29-23-18-13-34-14-19(18)23/h1,3-6,9-11,18-19,22-23,32H,2,7-8,12-14H2,(H,27,28,29)/t18-,19+,22-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094465
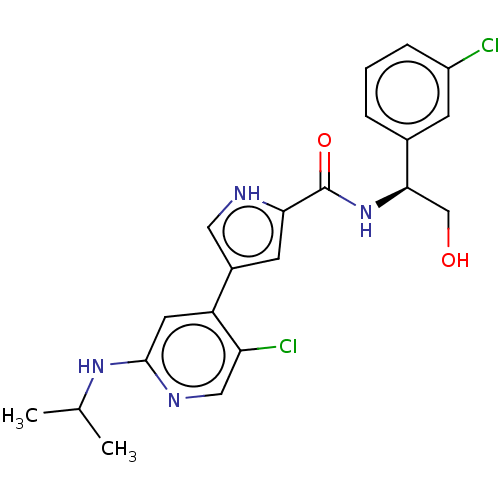
(CHEMBL3590106 | US10525036, Example BVD-523 | US10...)Show SMILES CC(C)Nc1cc(-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c(Cl)cn1 |r| Show InChI InChI=1S/C19H21NO3/c21-18(23-17-11-13-20-14-12-17)19(22,15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,17,20,22H,11-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115397
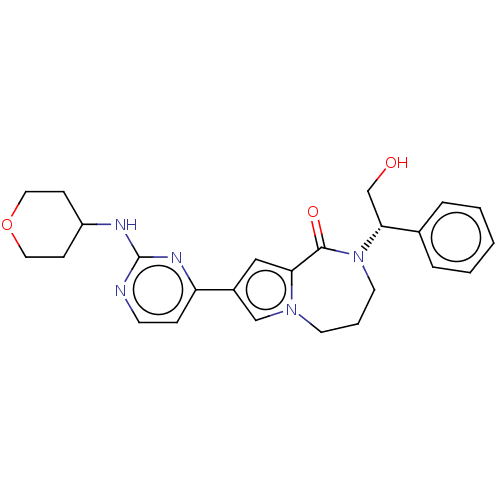
(CHEMBL3608462)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3/c31-17-23(18-5-2-1-3-6-18)30-12-4-11-29-16-19(15-22(29)24(30)32)21-7-10-26-25(28-21)27-20-8-13-33-14-9-20/h1-3,5-7,10,15-16,20,23,31H,4,8-9,11-14,17H2,(H,26,27,28)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115399
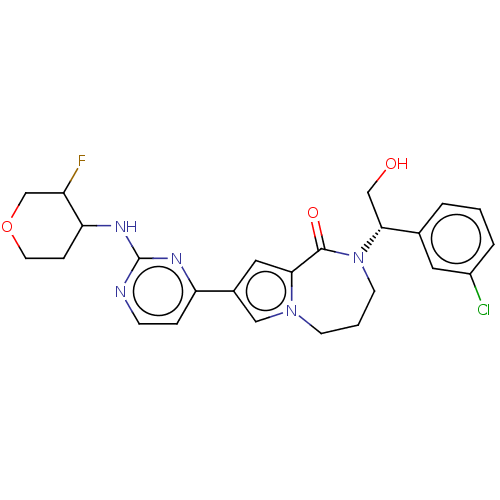
(CHEMBL3608464)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2F)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H27ClFN5O3/c26-18-4-1-3-16(11-18)23(14-33)32-9-2-8-31-13-17(12-22(31)24(32)34)20-5-7-28-25(29-20)30-21-6-10-35-15-19(21)27/h1,3-5,7,11-13,19,21,23,33H,2,6,8-10,14-15H2,(H,28,29,30)/t19?,21?,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115396
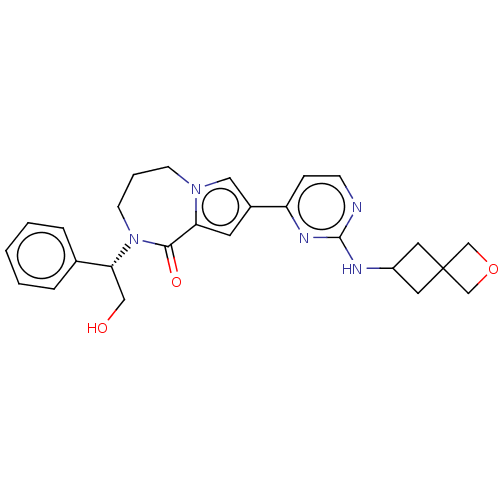
(CHEMBL3608463)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CC3(COC3)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C26H29N5O3/c32-15-23(18-5-2-1-3-6-18)31-10-4-9-30-14-19(11-22(30)24(31)33)21-7-8-27-25(29-21)28-20-12-26(13-20)16-34-17-26/h1-3,5-8,11,14,20,23,32H,4,9-10,12-13,15-17H2,(H,27,28,29)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115400

(CHEMBL3608587)Show SMILES OC[C@@H](N1C[C@H](F)Cn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H27ClFN5O3/c26-18-3-1-2-16(10-18)23(15-33)32-14-19(27)13-31-12-17(11-22(31)24(32)34)21-4-7-28-25(30-21)29-20-5-8-35-9-6-20/h1-4,7,10-12,19-20,23,33H,5-6,8-9,13-15H2,(H,28,29,30)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115403

(CHEMBL3608591)Show SMILES OC[C@@H](N1CCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C24H26ClN5O3/c25-18-3-1-2-16(12-18)22(15-31)30-9-8-29-14-17(13-21(29)23(30)32)20-4-7-26-24(28-20)27-19-5-10-33-11-6-19/h1-4,7,12-14,19,22,31H,5-6,8-11,15H2,(H,26,27,28)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115394

(CHEMBL3608461)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@H]2CC[C@@H](O)C2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H28ClN5O3/c26-18-4-1-3-16(11-18)23(15-32)31-10-2-9-30-14-17(12-22(30)24(31)34)21-7-8-27-25(29-21)28-19-5-6-20(33)13-19/h1,3-4,7-8,11-12,14,19-20,23,32-33H,2,5-6,9-10,13,15H2,(H,27,28,29)/t19-,20+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115393

(CHEMBL3608458)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)[C@@H](F)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c26-19-12-18(13-23(19)33)28-25-27-8-7-20(29-25)17-11-21-24(34)31(10-4-9-30(21)14-17)22(15-32)16-5-2-1-3-6-16/h1-3,5-8,11,14,18-19,22-23,32-33H,4,9-10,12-13,15H2,(H,27,28,29)/t18-,19-,22+,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115405
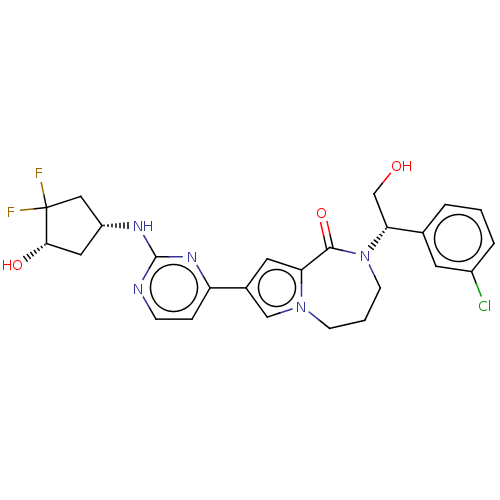
(CHEMBL3608459)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)C(F)(F)C2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26ClF2N5O3/c26-17-4-1-3-15(9-17)21(14-34)33-8-2-7-32-13-16(10-20(32)23(33)36)19-5-6-29-24(31-19)30-18-11-22(35)25(27,28)12-18/h1,3-6,9-10,13,18,21-22,34-35H,2,7-8,11-12,14H2,(H,29,30,31)/t18-,21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091509
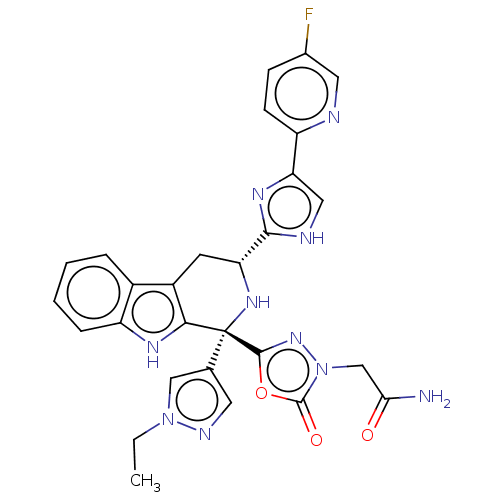
(CHEMBL3582344)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(CC(N)=O)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091503
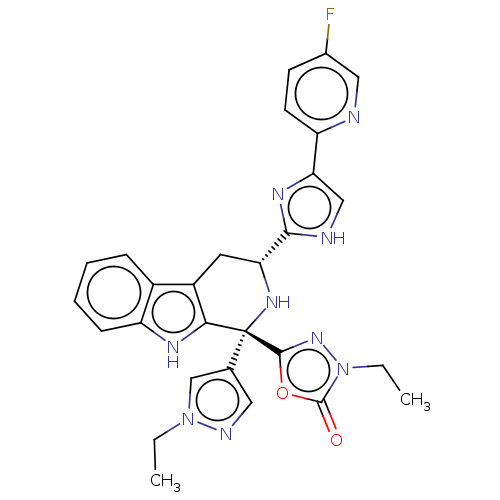
(CHEMBL3582339)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(CC)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091513
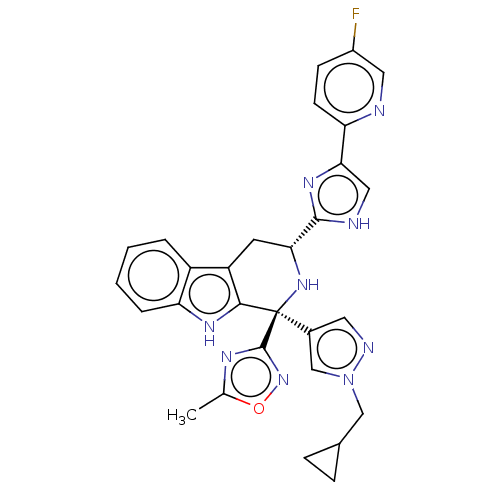
(CHEMBL3582321)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1cnn(CC2CC2)c1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091507
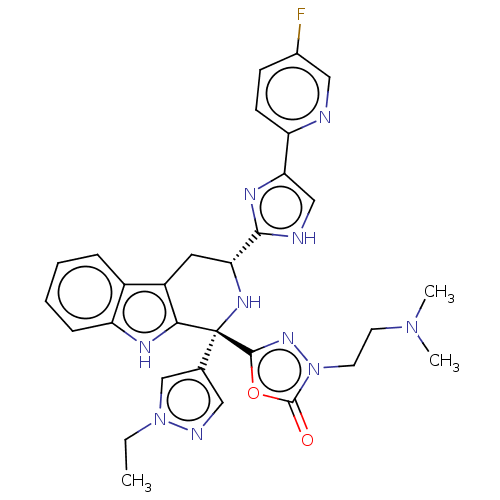
(CHEMBL3582342)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(CCN(C)C)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM50091414
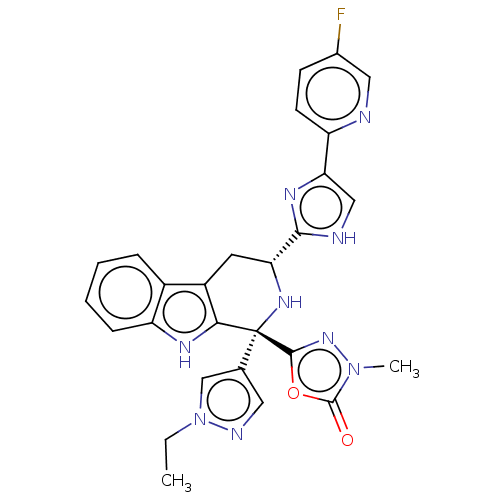
(CHEMBL3582336)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(C)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rat SSTR3 |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091420
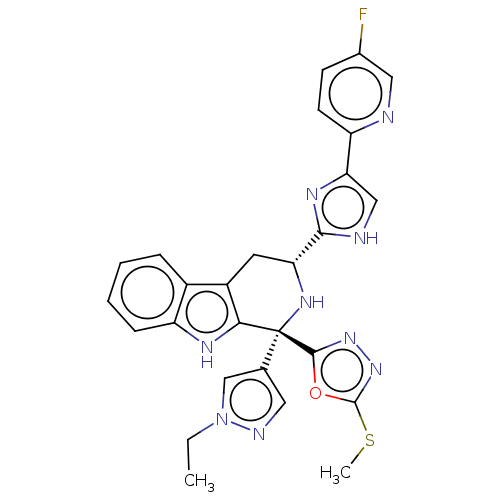
(CHEMBL3582329)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nnc(SC)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400517
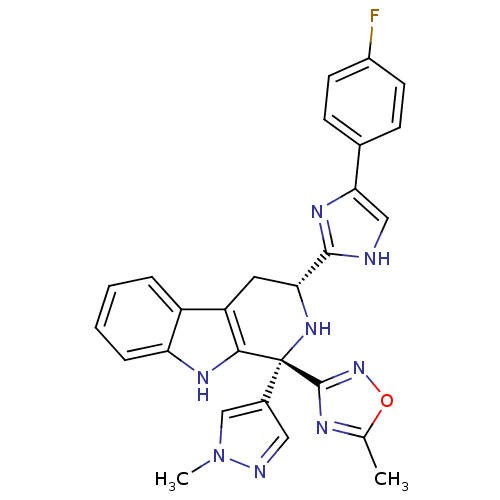
(CHEMBL2204935)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091508
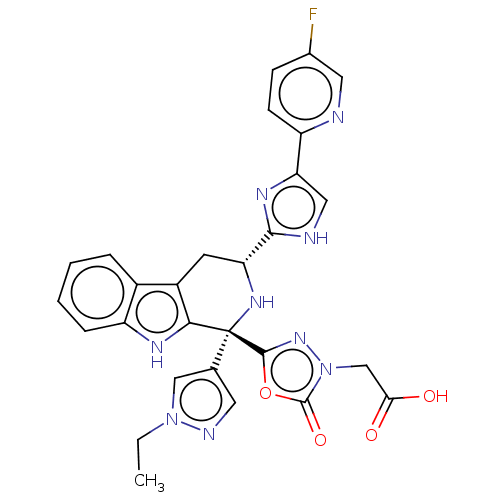
(CHEMBL3582343)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(CC(O)=O)c(=O)o1 |r| Show InChI InChI=1S/C37H60N4O16/c1-18(2)12-10-8-6-4-5-7-9-11-13-23(45)39-26-30(50)27(47)21(54-36(26)57-35-25(38-19(3)43)29(49)28(48)22(17-42)55-35)16-20(44)33-31(51)32(52)34(56-33)41-15-14-24(46)40-37(41)53/h11,13-15,18,20-22,25-36,42,44,47-52H,4-10,12,16-17H2,1-3H3,(H,38,43)(H,39,45)(H,40,46,53)/b13-11-/t20?,21-,22+,25+,26-,27+,28+,29+,30-,31+,32-,33?,34-,35+,36+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091498
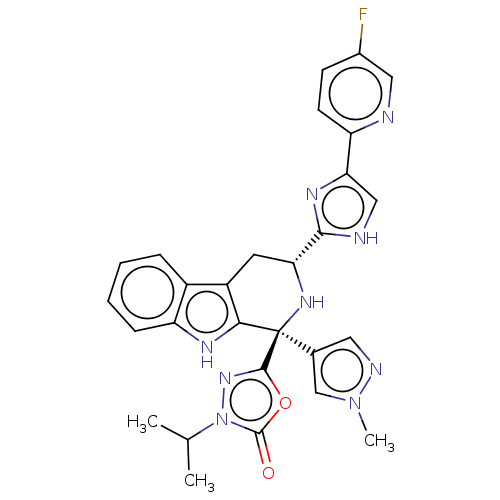
(CHEMBL3582335)Show SMILES CC(C)n1nc(oc1=O)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1cnn(C)c1 |r| Show InChI InChI=1S/C28H26FN9O2/c1-15(2)38-27(39)40-26(36-38)28(16-11-32-37(3)14-16)24-19(18-6-4-5-7-20(18)33-24)10-22(35-28)25-31-13-23(34-25)21-9-8-17(29)12-30-21/h4-9,11-15,22,33,35H,10H2,1-3H3,(H,31,34)/t22-,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389592

(CHEMBL2069500)Show SMILES C1CCC(CC1)C1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C26H28N4/c1-3-9-17(10-4-1)23-16-27-26(30-23)22-15-20-19-13-7-8-14-21(19)28-25(20)24(29-22)18-11-5-2-6-12-18/h1,3-4,7-10,13-14,16,18,22,24,28-29H,2,5-6,11-12,15H2,(H,27,30)/t22-,24?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50400517

(CHEMBL2204935)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091504
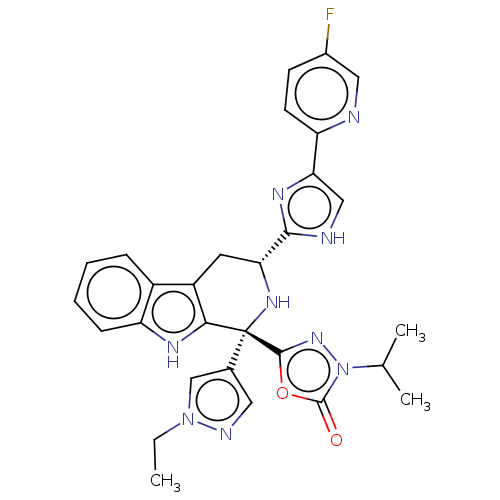
(CHEMBL3582340)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(C(C)C)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091513

(CHEMBL3582321)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1cnn(CC2CC2)c1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091414

(CHEMBL3582336)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(C)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091511
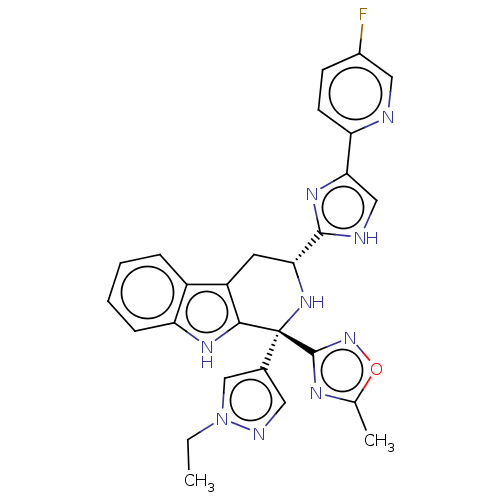
(CHEMBL3582319)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1noc(C)n1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091512
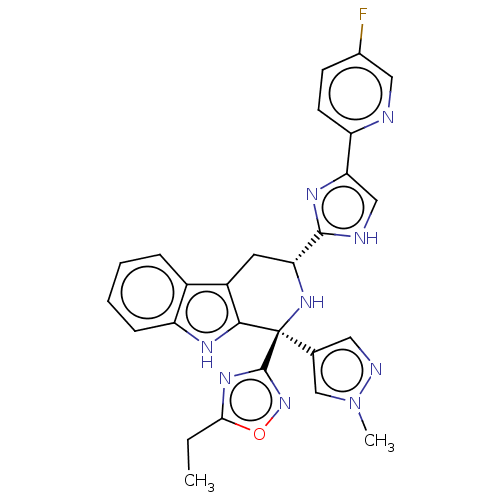
(CHEMBL3582320)Show SMILES CCc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1cnn(C)c1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091505
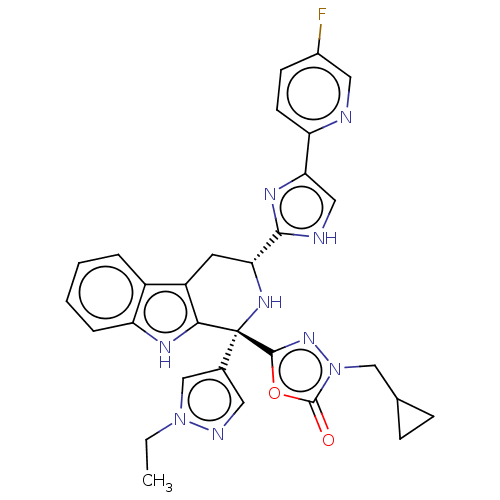
(CHEMBL3582341)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(CC2CC2)c(=O)o1 |r| Show InChI InChI=1S/C15H23N3O10/c16-3-5-8(21)11(24)14(26-5)27-6(4-19)12-9(22)10(23)13(28-12)18-2-1-7(20)17-15(18)25/h1-2,5-6,8-14,19,21-24H,3-4,16H2,(H,17,20,25)/t5-,6+,8-,9+,10-,11-,12-,13-,14+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50091414

(CHEMBL3582336)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(C)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from mouse recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091505

(CHEMBL3582341)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(CC2CC2)c(=O)o1 |r| Show InChI InChI=1S/C15H23N3O10/c16-3-5-8(21)11(24)14(26-5)27-6(4-19)12-9(22)10(23)13(28-12)18-2-1-7(20)17-15(18)25/h1-2,5-6,8-14,19,21-24H,3-4,16H2,(H,17,20,25)/t5-,6+,8-,9+,10-,11-,12-,13-,14+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091426
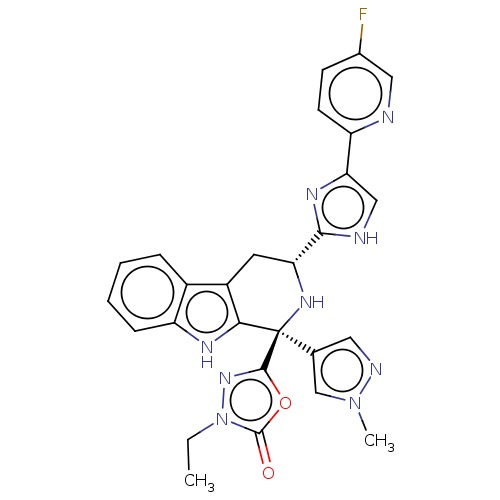
(CHEMBL3580681)Show SMILES CCn1nc(oc1=O)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1cnn(C)c1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091420

(CHEMBL3582329)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nnc(SC)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091498

(CHEMBL3582335)Show SMILES CC(C)n1nc(oc1=O)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1cnn(C)c1 |r| Show InChI InChI=1S/C28H26FN9O2/c1-15(2)38-27(39)40-26(36-38)28(16-11-32-37(3)14-16)24-19(18-6-4-5-7-20(18)33-24)10-22(35-28)25-31-13-23(34-25)21-9-8-17(29)12-30-21/h4-9,11-15,22,33,35H,10H2,1-3H3,(H,31,34)/t22-,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091507

(CHEMBL3582342)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(CCN(C)C)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091558

(CHEMBL3582311)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)C(N1)(c1ccccc1)C(F)(F)F |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091504

(CHEMBL3582340)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(C(C)C)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091422
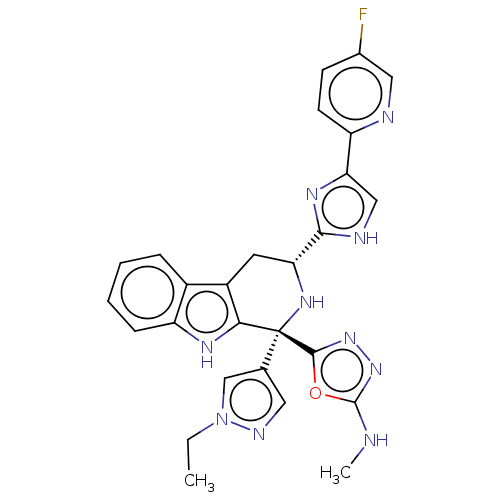
(CHEMBL3582331)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nnc(NC)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091417
(CHEMBL3582326)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nnc(C)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091511

(CHEMBL3582319)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1noc(C)n1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115395

(CHEMBL3608460)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@H]2CC[C@@H](O)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3/c31-16-23(17-5-2-1-3-6-17)30-12-4-11-29-15-18(13-22(29)24(30)33)21-9-10-26-25(28-21)27-19-7-8-20(32)14-19/h1-3,5-6,9-10,13,15,19-20,23,31-32H,4,7-8,11-12,14,16H2,(H,26,27,28)/t19-,20+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091414

(CHEMBL3582336)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(C)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50091509

(CHEMBL3582344)Show SMILES CCn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cn1)c1nn(CC(N)=O)c(=O)o1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human recombinant SSTR3 expressed in CHO cell membranes incubated for 60 to 90 mins by radioligand binding assay |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50054480

(CHEMBL3323086)Show SMILES Cn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1ccc(cn1)C(O)=O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant SSTR3 expressed in CHO cells assessed as reduction in forskolin-induced cAMP accumulation in presence o... |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data