 Found 1572 hits with Last Name = 'feng' and Initial = 'z'
Found 1572 hits with Last Name = 'feng' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389603

(CHEMBL2069499)Show SMILES CCCCC1(CCCC)N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SST3 receptor expressed in CHO cells after 60 mins by scintillation counting |
ACS Med Chem Lett 3: 289-293 (2012)
Article DOI: 10.1021/ml200272z
BindingDB Entry DOI: 10.7270/Q2K938MH |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389592
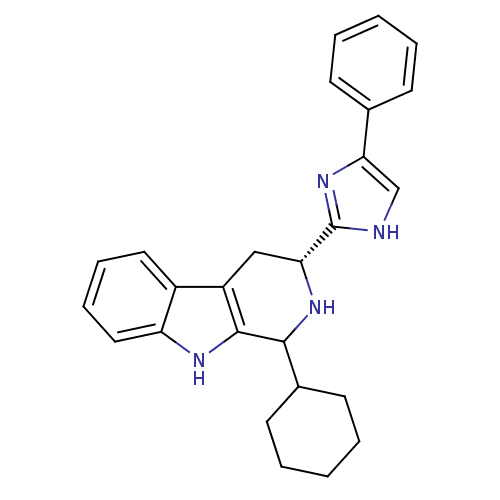
(CHEMBL2069500)Show SMILES C1CCC(CC1)C1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C26H28N4/c1-3-9-17(10-4-1)23-16-27-26(30-23)22-15-20-19-13-7-8-14-21(19)28-25(20)24(29-22)18-11-5-2-6-12-18/h1,3-4,7-10,13-14,16,18,22,24,28-29H,2,5-6,11-12,15H2,(H,27,30)/t22-,24?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human SST3 receptor |
ACS Med Chem Lett 3: 289-293 (2012)
Article DOI: 10.1021/ml200272z
BindingDB Entry DOI: 10.7270/Q2K938MH |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM150169
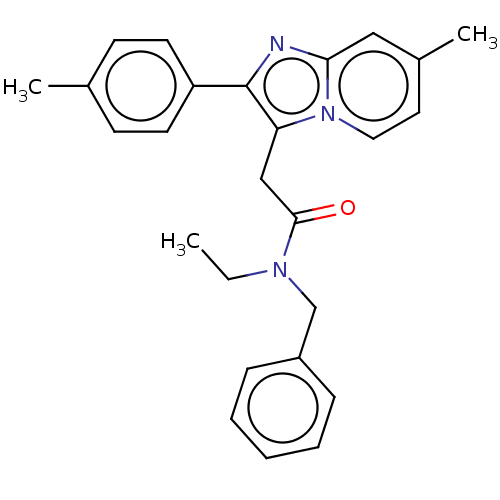
(US8980887, Compound 13)Show SMILES CCN(Cc1ccccc1)C(=O)Cc1c(nc2cc(C)ccn12)-c1ccc(C)cc1 Show InChI InChI=1S/C26H27N3O/c1-4-28(18-21-8-6-5-7-9-21)25(30)17-23-26(22-12-10-19(2)11-13-22)27-24-16-20(3)14-15-29(23)24/h5-16H,4,17-18H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.97 | -49.5 | 6.99 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China
US Patent
| Assay Description
Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... |
US Patent US8980887 (2015)
BindingDB Entry DOI: 10.7270/Q2D50KP2 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM150168
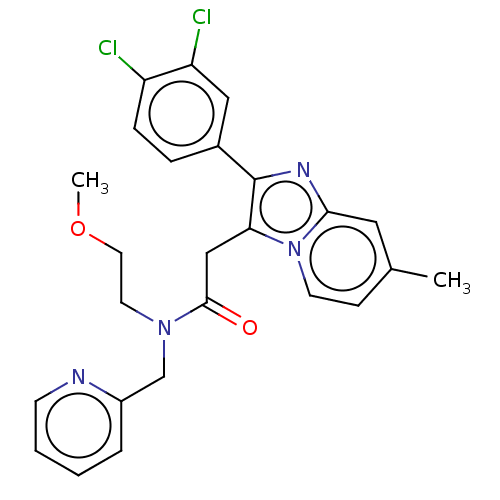
(US8980887, Compound 11)Show SMILES COCCN(Cc1ccccn1)C(=O)Cc1c(nc2cc(C)ccn12)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H24Cl2N4O2/c1-17-8-10-31-22(25(29-23(31)13-17)18-6-7-20(26)21(27)14-18)15-24(32)30(11-12-33-2)16-19-5-3-4-9-28-19/h3-10,13-14H,11-12,15-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 6.66 | -47.5 | 15.6 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China
US Patent
| Assay Description
Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... |
US Patent US8980887 (2015)
BindingDB Entry DOI: 10.7270/Q2D50KP2 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM150164
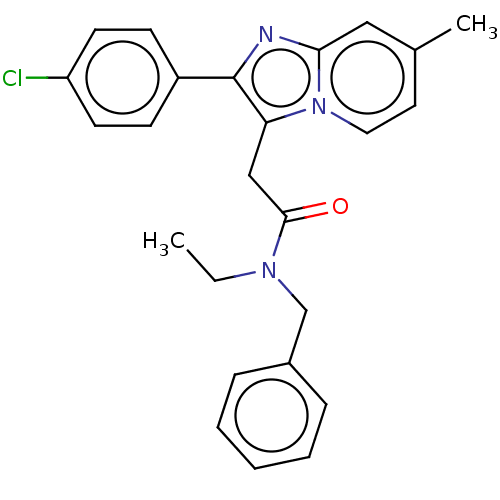
(US8980887, Compound 1)Show SMILES CCN(Cc1ccccc1)C(=O)Cc1c(nc2cc(C)ccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H24ClN3O/c1-3-28(17-19-7-5-4-6-8-19)24(30)16-22-25(20-9-11-21(26)12-10-20)27-23-15-18(2)13-14-29(22)23/h4-15H,3,16-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 7.17 | -47.3 | 16.9 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China
US Patent
| Assay Description
Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... |
US Patent US8980887 (2015)
BindingDB Entry DOI: 10.7270/Q2D50KP2 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM150166
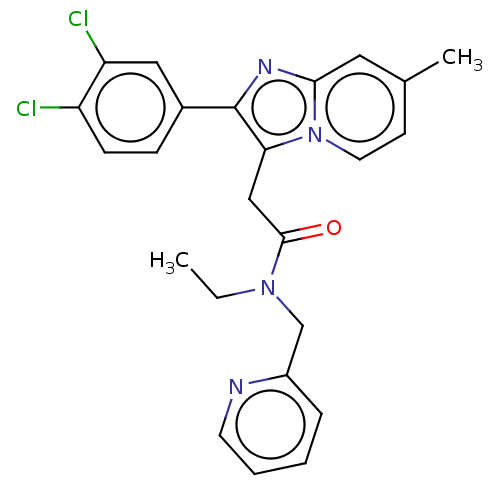
(US8980887, Compound 8)Show SMILES CCN(Cc1ccccn1)C(=O)Cc1c(nc2cc(C)ccn12)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C24H22Cl2N4O/c1-3-29(15-18-6-4-5-10-27-18)23(31)14-21-24(17-7-8-19(25)20(26)13-17)28-22-12-16(2)9-11-30(21)22/h4-13H,3,14-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 9.34 | -46.6 | 21.9 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China
US Patent
| Assay Description
Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... |
US Patent US8980887 (2015)
BindingDB Entry DOI: 10.7270/Q2D50KP2 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM150167
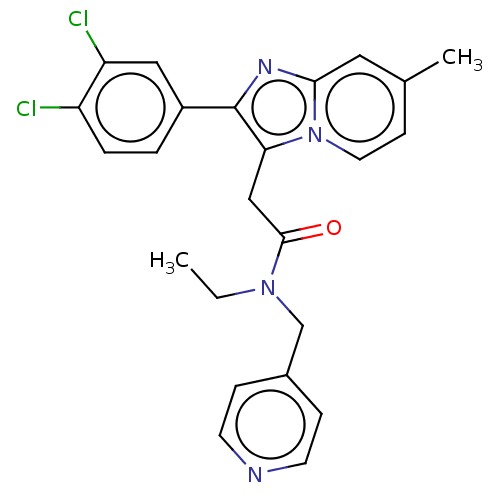
(US8980887, Compound 10)Show SMILES CCN(Cc1ccncc1)C(=O)Cc1c(nc2cc(C)ccn12)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C24H22Cl2N4O/c1-3-29(15-17-6-9-27-10-7-17)23(31)14-21-24(18-4-5-19(25)20(26)13-18)28-22-12-16(2)8-11-30(21)22/h4-13H,3,14-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 11.1 | -46.2 | 26.1 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China
US Patent
| Assay Description
Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... |
US Patent US8980887 (2015)
BindingDB Entry DOI: 10.7270/Q2D50KP2 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142238
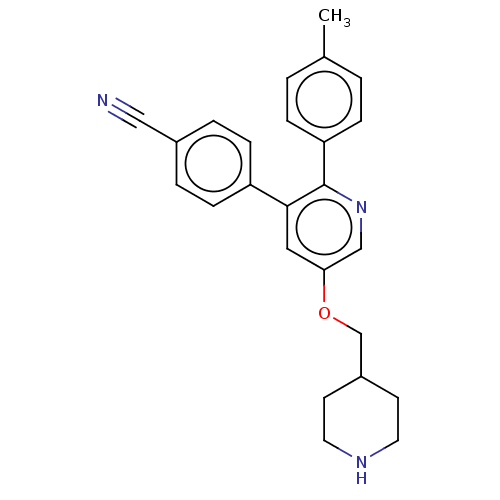
(CHEMBL3759201)Show SMILES Cc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H25N3O/c1-18-2-6-22(7-3-18)25-24(21-8-4-19(15-26)5-9-21)14-23(16-28-25)29-17-20-10-12-27-13-11-20/h2-9,14,16,20,27H,10-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Translocator protein
(Rattus norvegicus (rat)) | BDBM150170
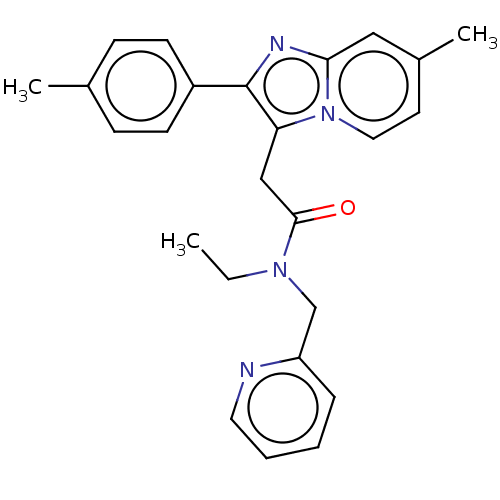
(US8980887, Compound 14)Show SMILES CCN(Cc1ccccn1)C(=O)Cc1c(nc2cc(C)ccn12)-c1ccc(C)cc1 Show InChI InChI=1S/C25H26N4O/c1-4-28(17-21-7-5-6-13-26-21)24(30)16-22-25(20-10-8-18(2)9-11-20)27-23-15-19(3)12-14-29(22)23/h5-15H,4,16-17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 38.7 | -43.0 | 91.0 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China
US Patent
| Assay Description
Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... |
US Patent US8980887 (2015)
BindingDB Entry DOI: 10.7270/Q2D50KP2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400528
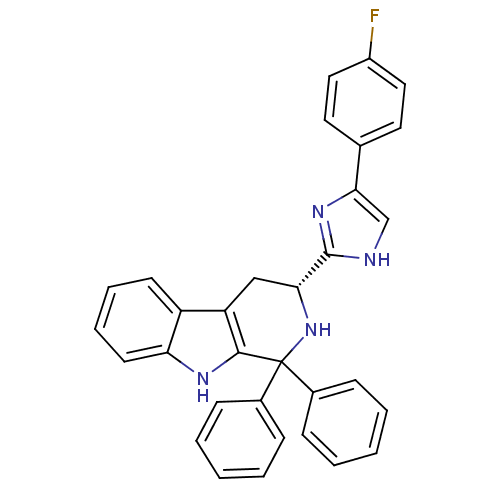
(CHEMBL2204934)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)C(N1)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C32H25FN4/c33-24-17-15-21(16-18-24)29-20-34-31(36-29)28-19-26-25-13-7-8-14-27(25)35-30(26)32(37-28,22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-18,20,28,35,37H,19H2,(H,34,36)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142187

(CHEMBL3758634)Show SMILES FC(F)(F)Oc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H22F3N3O2/c26-25(27,28)33-21-7-5-20(6-8-21)24-23(19-3-1-17(14-29)2-4-19)13-22(15-31-24)32-16-18-9-11-30-12-10-18/h1-8,13,15,18,30H,9-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM150165
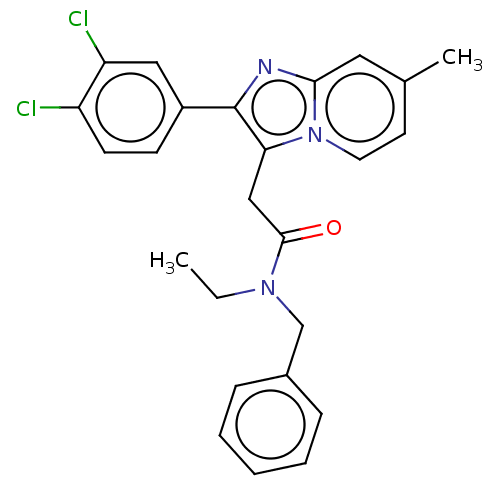
(US8980887, Compound 7)Show SMILES CCN(Cc1ccccc1)C(=O)Cc1c(nc2cc(C)ccn12)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H23Cl2N3O/c1-3-29(16-18-7-5-4-6-8-18)24(31)15-22-25(19-9-10-20(26)21(27)14-19)28-23-13-17(2)11-12-30(22)23/h4-14H,3,15-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 49.2 | -42.4 | 116 | n/a | n/a | n/a | n/a | n/a | 30 |
Institute of Pharmacology; Toxicology Academy of Military Medical Sciences P.L.A., China
US Patent
| Assay Description
Competitive Binding Test of Drug to the Receptor (Rat Heart TSPO) and Radioligand (3H-PK11195): (1) tubes were placed in reaction condition of 30 C.(... |
US Patent US8980887 (2015)
BindingDB Entry DOI: 10.7270/Q2D50KP2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50091554
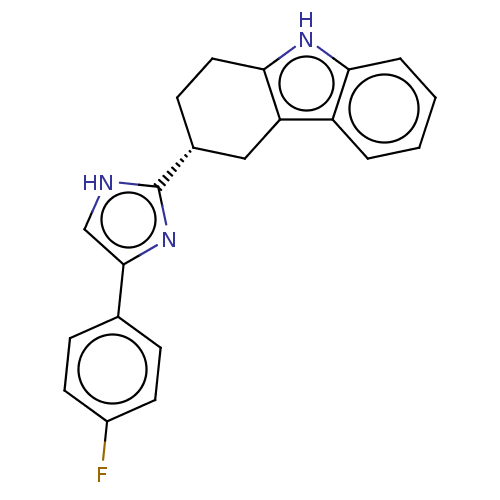
(CHEMBL3582308)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@@H]1CCc2[nH]c3ccccc3c2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142237
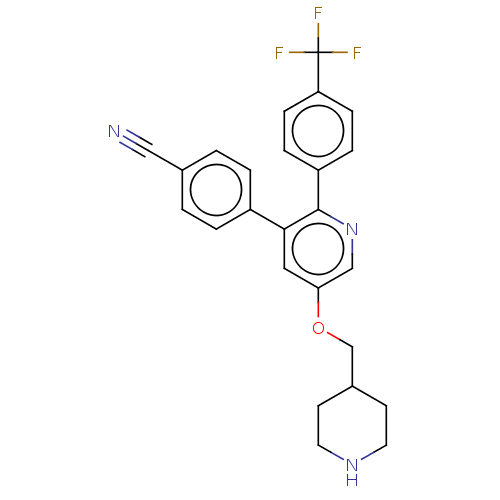
(CHEMBL3759797)Show SMILES FC(F)(F)c1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H22F3N3O/c26-25(27,28)21-7-5-20(6-8-21)24-23(19-3-1-17(14-29)2-4-19)13-22(15-31-24)32-16-18-9-11-30-12-10-18/h1-8,13,15,18,30H,9-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(BOVINE) | BDBM50247862

(5Z-(9S,11R,15S)-13-Oxa-17-(3-trifluoromethyl)pheny...)Show SMILES O[C@@H](CCc1cccc(c1)C(F)(F)F)CO[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O |r| Show InChI InChI=1S/C23H31F3O6/c24-23(25,26)16-7-5-6-15(12-16)10-11-17(27)14-32-22-18(19(28)13-20(22)29)8-3-1-2-4-9-21(30)31/h1,3,5-7,12,17-20,22,27-29H,2,4,8-11,13-14H2,(H,30,31)/b3-1-/t17-,18-,19-,20+,22+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF2 alpha from FP receptor in bovine corpus luteum membrane |
Bioorg Med Chem 17: 576-84 (2009)
Article DOI: 10.1016/j.bmc.2008.11.070
BindingDB Entry DOI: 10.7270/Q2RJ4JC1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50389592

(CHEMBL2069500)Show SMILES C1CCC(CC1)C1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C26H28N4/c1-3-9-17(10-4-1)23-16-27-26(30-23)22-15-20-19-13-7-8-14-21(19)28-25(20)24(29-22)18-11-5-2-6-12-18/h1,3-4,7-10,13-14,16,18,22,24,28-29H,2,5-6,11-12,15H2,(H,27,30)/t22-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(BOVINE) | BDBM50247917

(5Z-(9S,11R,15S)-13-Oxa-16-phenoxy-propoxy]-9,11,15...)Show SMILES O[C@@H](CO[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O)COc1ccccc1 |r| Show InChI InChI=1S/C21H30O7/c22-15(13-27-16-8-4-3-5-9-16)14-28-21-17(18(23)12-19(21)24)10-6-1-2-7-11-20(25)26/h1,3-6,8-9,15,17-19,21-24H,2,7,10-14H2,(H,25,26)/b6-1-/t15-,17+,18+,19-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF2 alpha from FP receptor in bovine corpus luteum membrane |
Bioorg Med Chem 17: 576-84 (2009)
Article DOI: 10.1016/j.bmc.2008.11.070
BindingDB Entry DOI: 10.7270/Q2RJ4JC1 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142242
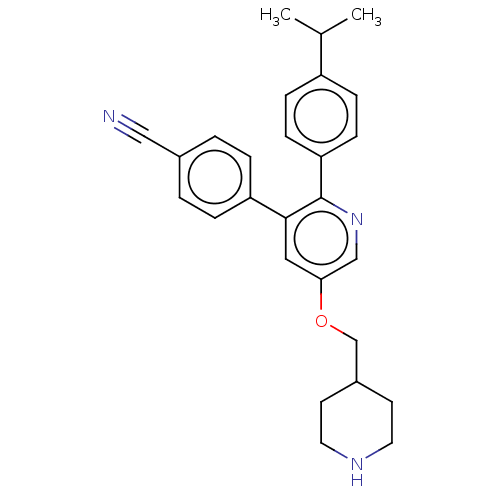
(CHEMBL3759102)Show SMILES CC(C)c1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C27H29N3O/c1-19(2)22-7-9-24(10-8-22)27-26(23-5-3-20(16-28)4-6-23)15-25(17-30-27)31-18-21-11-13-29-14-12-21/h3-10,15,17,19,21,29H,11-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(BOVINE) | BDBM50423647

(LANATOPROST | Latanoprost | PHXA-41 | PHXA41 | XA-...)Show SMILES CC(C)OC(=O)CCC\C=C/C[C@@H]1[C@H](O)C[C@H](O)[C@H]1CC[C@@H](O)CCc1ccccc1 |r| Show InChI InChI=1S/C26H40O5/c1-19(2)31-26(30)13-9-4-3-8-12-22-23(25(29)18-24(22)28)17-16-21(27)15-14-20-10-6-5-7-11-20/h3,5-8,10-11,19,21-25,27-29H,4,9,12-18H2,1-2H3/b8-3-/t21-,22-,23-,24+,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF2 alpha from FP receptor in bovine corpus luteum membrane |
Bioorg Med Chem 17: 576-84 (2009)
Article DOI: 10.1016/j.bmc.2008.11.070
BindingDB Entry DOI: 10.7270/Q2RJ4JC1 |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(BOVINE) | BDBM50247915
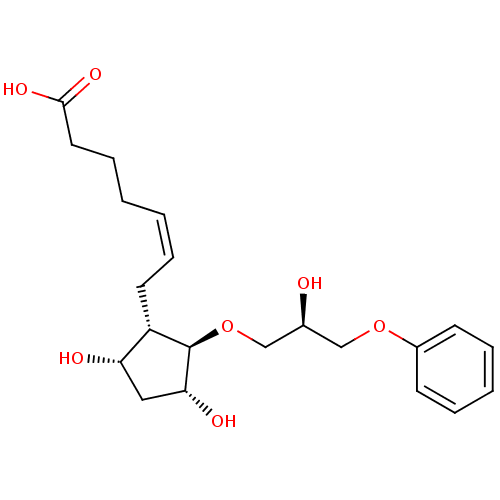
(5Z-(9S,11R,15R)-13-Oxa-16-phenoxy-propoxy]-9,11,15...)Show SMILES O[C@H](CO[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O)COc1ccccc1 |r| Show InChI InChI=1S/C21H30O7/c22-15(13-27-16-8-4-3-5-9-16)14-28-21-17(18(23)12-19(21)24)10-6-1-2-7-11-20(25)26/h1,3-6,8-9,15,17-19,21-24H,2,7,10-14H2,(H,25,26)/b6-1-/t15-,17-,18-,19+,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF2 alpha from FP receptor in bovine corpus luteum membrane |
Bioorg Med Chem 17: 576-84 (2009)
Article DOI: 10.1016/j.bmc.2008.11.070
BindingDB Entry DOI: 10.7270/Q2RJ4JC1 |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(BOVINE) | BDBM50247863
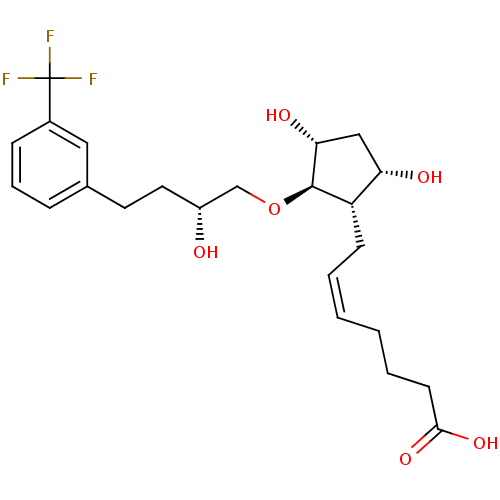
(5Z-(9S,11R,15R)-13-Oxa-17-(3-trifluoromethyl)pheny...)Show SMILES O[C@H](CCc1cccc(c1)C(F)(F)F)CO[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O |r| Show InChI InChI=1S/C23H31F3O6/c24-23(25,26)16-7-5-6-15(12-16)10-11-17(27)14-32-22-18(19(28)13-20(22)29)8-3-1-2-4-9-21(30)31/h1,3,5-7,12,17-20,22,27-29H,2,4,8-11,13-14H2,(H,30,31)/b3-1-/t17-,18+,19+,20-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF2 alpha from FP receptor in bovine corpus luteum membrane |
Bioorg Med Chem 17: 576-84 (2009)
Article DOI: 10.1016/j.bmc.2008.11.070
BindingDB Entry DOI: 10.7270/Q2RJ4JC1 |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(BOVINE) | BDBM50035622
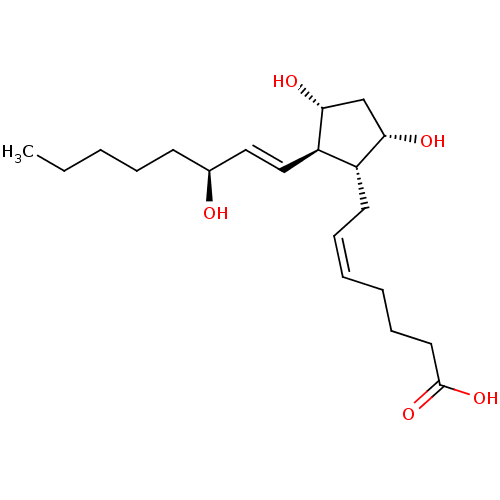
((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-19,21-23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF2 alpha from FP receptor in bovine corpus luteum membrane |
Bioorg Med Chem 17: 576-84 (2009)
Article DOI: 10.1016/j.bmc.2008.11.070
BindingDB Entry DOI: 10.7270/Q2RJ4JC1 |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(BOVINE) | BDBM50247918

(5Z-(9S,11R,15S)-13-Oxa-16-(3-chloro)phenoxy-propox...)Show SMILES O[C@@H](CO[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O)COc1cccc(Cl)c1 |r| Show InChI InChI=1S/C21H29ClO7/c22-14-6-5-7-16(10-14)28-12-15(23)13-29-21-17(18(24)11-19(21)25)8-3-1-2-4-9-20(26)27/h1,3,5-7,10,15,17-19,21,23-25H,2,4,8-9,11-13H2,(H,26,27)/b3-1-/t15-,17+,18+,19-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF2 alpha from FP receptor in bovine corpus luteum membrane |
Bioorg Med Chem 17: 576-84 (2009)
Article DOI: 10.1016/j.bmc.2008.11.070
BindingDB Entry DOI: 10.7270/Q2RJ4JC1 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50139118
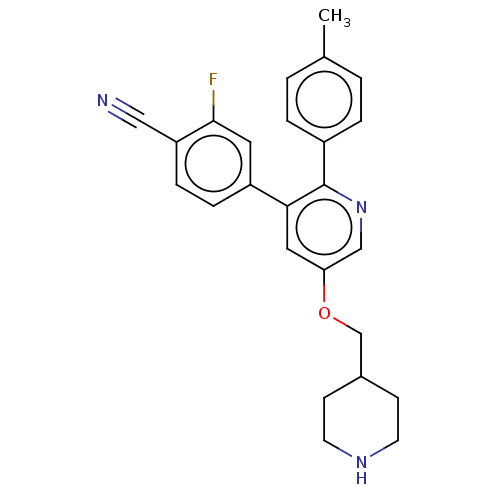
(CHEMBL3759351)Show SMILES Cc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(C#N)c(F)c1 Show InChI InChI=1S/C25H24FN3O/c1-17-2-4-19(5-3-17)25-23(20-6-7-21(14-27)24(26)12-20)13-22(15-29-25)30-16-18-8-10-28-11-9-18/h2-7,12-13,15,18,28H,8-11,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142240
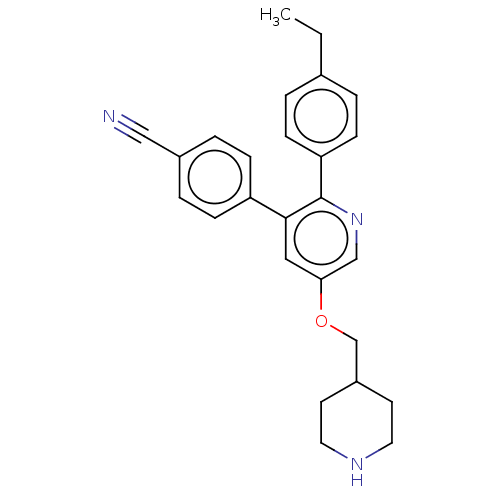
(CHEMBL3759861)Show SMILES CCc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C26H27N3O/c1-2-19-3-9-23(10-4-19)26-25(22-7-5-20(16-27)6-8-22)15-24(17-29-26)30-18-21-11-13-28-14-12-21/h3-10,15,17,21,28H,2,11-14,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50139119
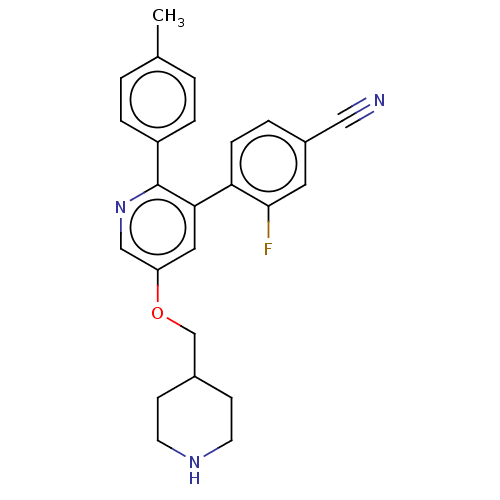
(CHEMBL3758849)Show SMILES Cc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1F)C#N Show InChI InChI=1S/C25H24FN3O/c1-17-2-5-20(6-3-17)25-23(22-7-4-19(14-27)12-24(22)26)13-21(15-29-25)30-16-18-8-10-28-11-9-18/h2-7,12-13,15,18,28H,8-11,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142249
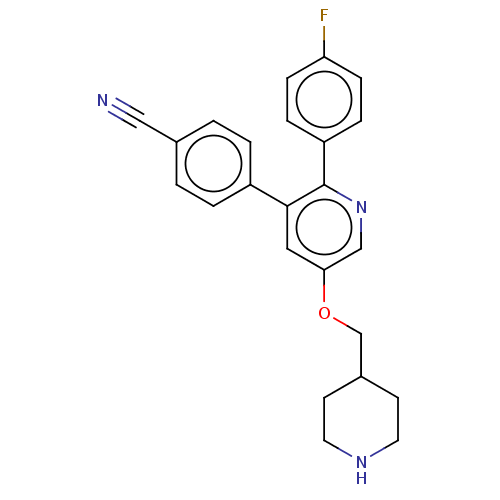
(CHEMBL3759939)Show SMILES Fc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C24H22FN3O/c25-21-7-5-20(6-8-21)24-23(19-3-1-17(14-26)2-4-19)13-22(15-28-24)29-16-18-9-11-27-12-10-18/h1-8,13,15,18,27H,9-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50091555
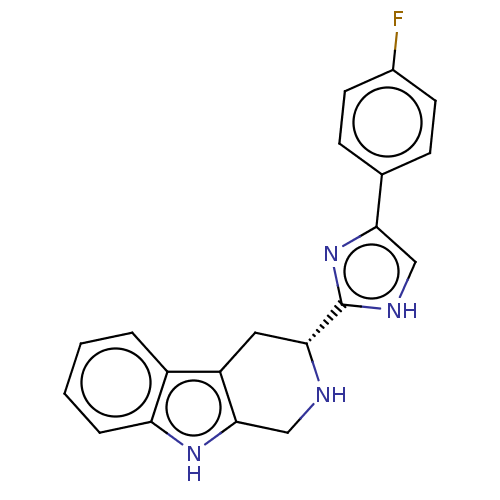
(CHEMBL3582307)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c(CN1)[nH]c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400518

(CHEMBL2204942)Show SMILES Cn1cc(cn1)[C@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H21FN6/c1-31-13-15(11-27-31)22-23-18(17-4-2-3-5-19(17)28-23)10-20(29-22)24-26-12-21(30-24)14-6-8-16(25)9-7-14/h2-9,11-13,20,22,28-29H,10H2,1H3,(H,26,30)/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50389590
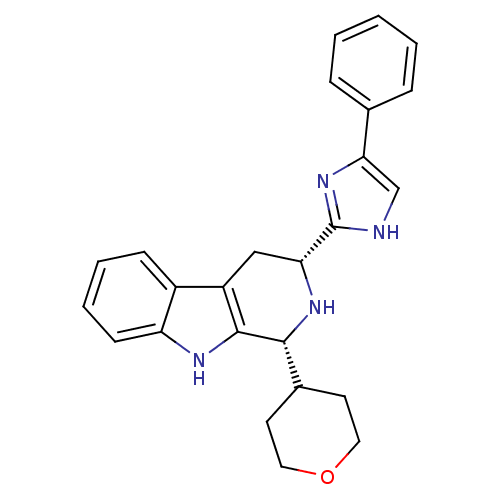
(CHEMBL2069502)Show SMILES C1CC(CCO1)[C@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C25H26N4O/c1-2-6-16(7-3-1)22-15-26-25(29-22)21-14-19-18-8-4-5-9-20(18)27-24(19)23(28-21)17-10-12-30-13-11-17/h1-9,15,17,21,23,27-28H,10-14H2,(H,26,29)/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400519

(CHEMBL2204941)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)[C@H](N1)C1CCOCC1 |r| Show InChI InChI=1S/C25H25FN4O/c26-17-7-5-15(6-8-17)22-14-27-25(30-22)21-13-19-18-3-1-2-4-20(18)28-24(19)23(29-21)16-9-11-31-12-10-16/h1-8,14,16,21,23,28-29H,9-13H2,(H,27,30)/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142079
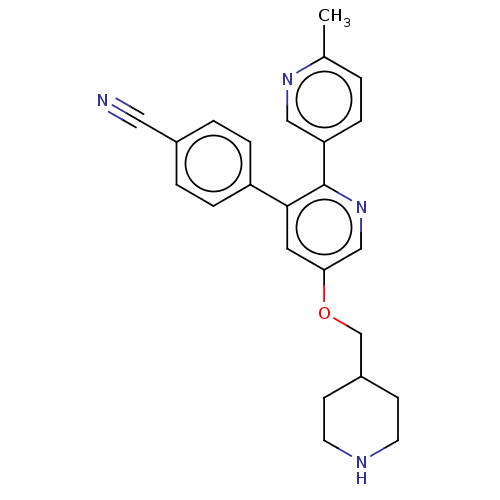
(CHEMBL3758636)Show SMILES Cc1ccc(cn1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C24H24N4O/c1-17-2-5-21(14-27-17)24-23(20-6-3-18(13-25)4-7-20)12-22(15-28-24)29-16-19-8-10-26-11-9-19/h2-7,12,14-15,19,26H,8-11,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400520

(CHEMBL2204932)Show SMILES Cc1nc(no1)[C@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H19FN6O/c1-12-26-23(30-31-12)21-20-16(15-4-2-3-5-17(15)27-20)10-18(28-21)22-25-11-19(29-22)13-6-8-14(24)9-7-13/h2-9,11,18,21,27-28H,10H2,1H3,(H,25,29)/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50091553
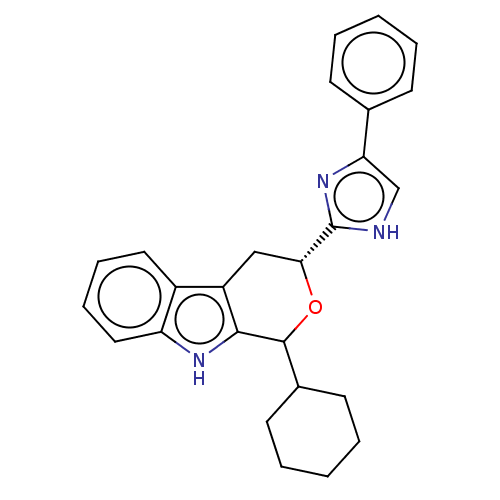
(CHEMBL3582309)Show SMILES C1CCC(CC1)C1O[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400526

(CHEMBL2204937)Show SMILES Cc1nc(no1)[C@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM554543
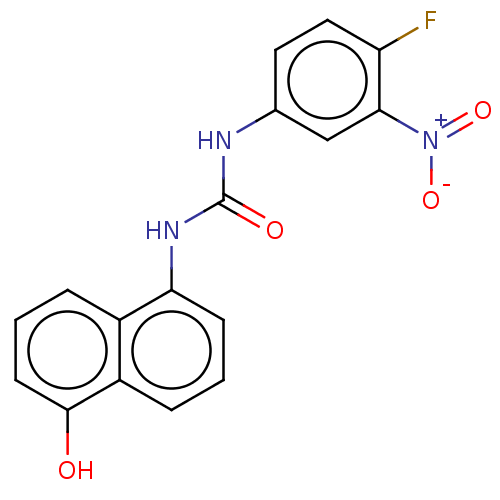
(US11339122, Compound 14)Show SMILES Oc1cccc2c(NC(=O)Nc3ccc(F)c(c3)[N+]([O-])=O)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding studies with [3H]RTX were carried out as follows. The binding assay mixtures were prepared in 1.5 ml centrifuge tubes and consisted of a fixe... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21839QZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM554544
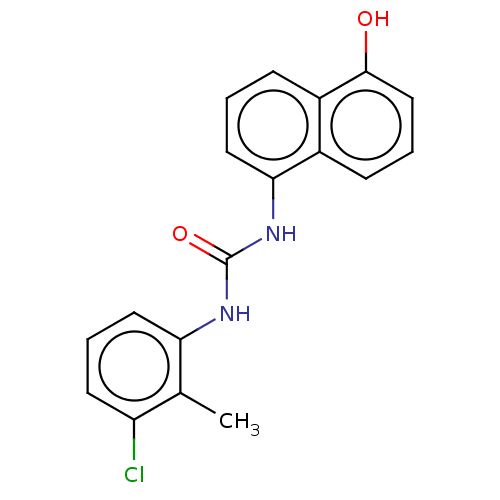
(US11339122, Compound 15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding studies with [3H]RTX were carried out as follows. The binding assay mixtures were prepared in 1.5 ml centrifuge tubes and consisted of a fixe... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21839QZ |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142086
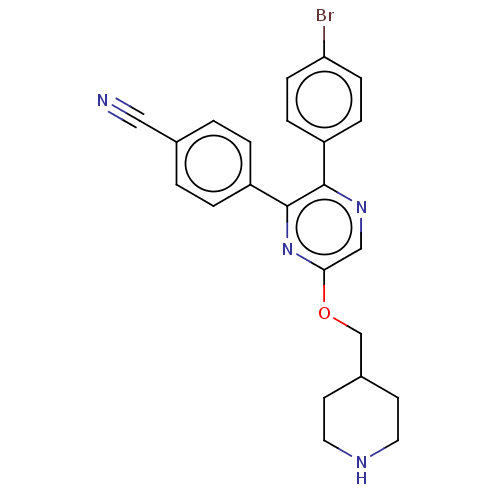
(CHEMBL3758396)Show SMILES Brc1ccc(cc1)-c1ncc(OCC2CCNCC2)nc1-c1ccc(cc1)C#N Show InChI InChI=1S/C23H21BrN4O/c24-20-7-5-18(6-8-20)22-23(19-3-1-16(13-25)2-4-19)28-21(14-27-22)29-15-17-9-11-26-12-10-17/h1-8,14,17,26H,9-12,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400525
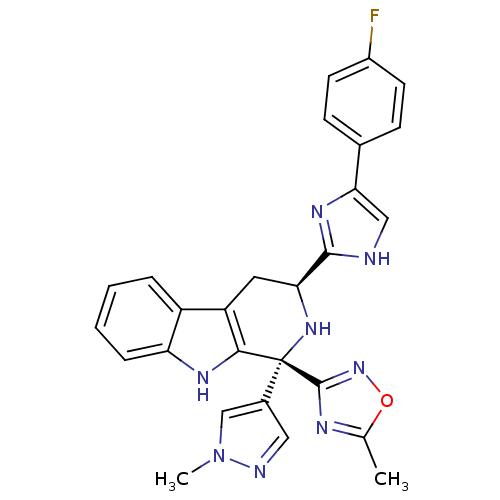
(CHEMBL2204938)Show SMILES Cc1nc(no1)[C@@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM554545
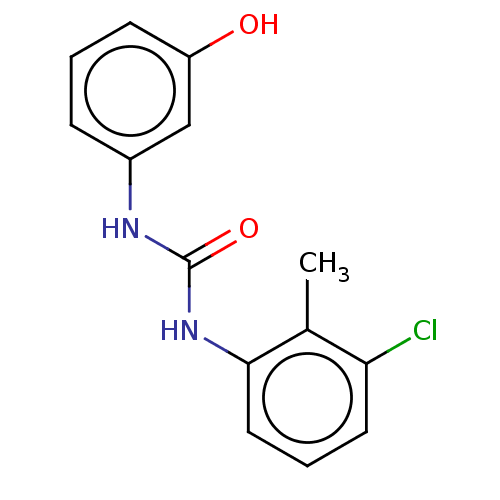
(US11339122, Compound 16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding studies with [3H]RTX were carried out as follows. The binding assay mixtures were prepared in 1.5 ml centrifuge tubes and consisted of a fixe... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21839QZ |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142235

(CHEMBL3758821)Show SMILES Fc1ccc(cc1F)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C24H21F2N3O/c25-22-6-5-19(11-23(22)26)24-21(18-3-1-16(13-27)2-4-18)12-20(14-29-24)30-15-17-7-9-28-10-8-17/h1-6,11-12,14,17,28H,7-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142243
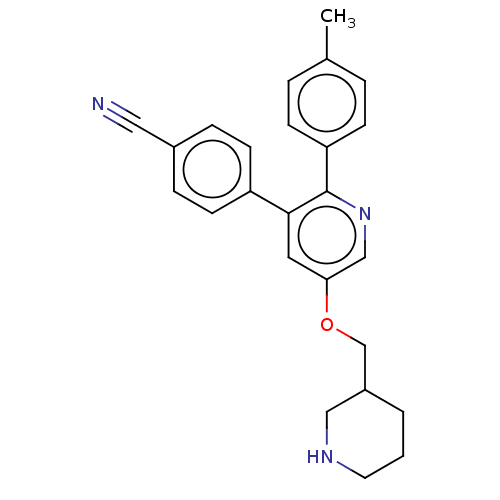
(CHEMBL3758340)Show SMILES Cc1ccc(cc1)-c1ncc(OCC2CCCNC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H25N3O/c1-18-4-8-22(9-5-18)25-24(21-10-6-19(14-26)7-11-21)13-23(16-28-25)29-17-20-3-2-12-27-15-20/h4-11,13,16,20,27H,2-3,12,15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(BOVINE) | BDBM50247916
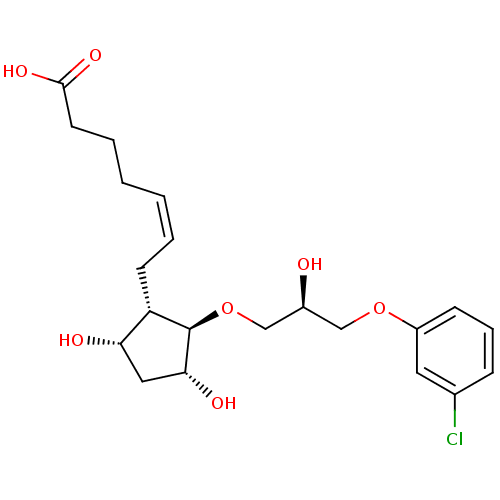
(5Z-(9S,11R,15R)-13-Oxa-16-(3-chloro)phenoxy-propox...)Show SMILES O[C@H](CO[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O)COc1cccc(Cl)c1 |r| Show InChI InChI=1S/C21H29ClO7/c22-14-6-5-7-16(10-14)28-12-15(23)13-29-21-17(18(24)11-19(21)25)8-3-1-2-4-9-20(26)27/h1,3,5-7,10,15,17-19,21,23-25H,2,4,8-9,11-13H2,(H,26,27)/b3-1-/t15-,17-,18-,19+,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF2 alpha from FP receptor in bovine corpus luteum membrane |
Bioorg Med Chem 17: 576-84 (2009)
Article DOI: 10.1016/j.bmc.2008.11.070
BindingDB Entry DOI: 10.7270/Q2RJ4JC1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50091558

(CHEMBL3582311)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)C(N1)(c1ccccc1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
ACS Med Chem Lett 6: 513-7 (2015)
Article DOI: 10.1021/ml500514w
BindingDB Entry DOI: 10.7270/Q2M61N0H |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142185
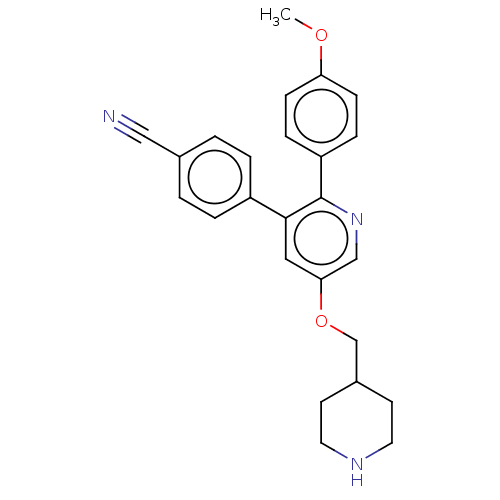
(CHEMBL3759654)Show SMILES COc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H25N3O2/c1-29-22-8-6-21(7-9-22)25-24(20-4-2-18(15-26)3-5-20)14-23(16-28-25)30-17-19-10-12-27-13-11-19/h2-9,14,16,19,27H,10-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142239
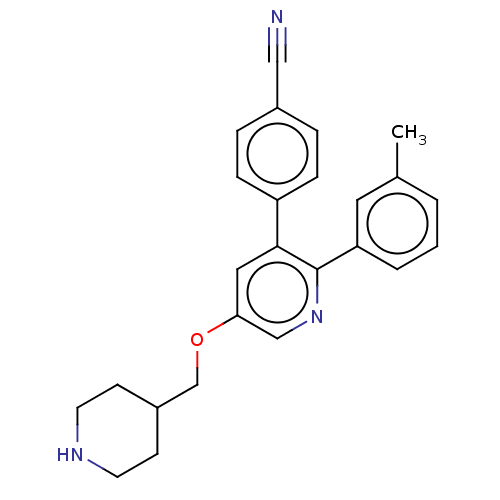
(CHEMBL3760120)Show SMILES Cc1cccc(c1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H25N3O/c1-18-3-2-4-22(13-18)25-24(21-7-5-19(15-26)6-8-21)14-23(16-28-25)29-17-20-9-11-27-12-10-20/h2-8,13-14,16,20,27H,9-12,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142236
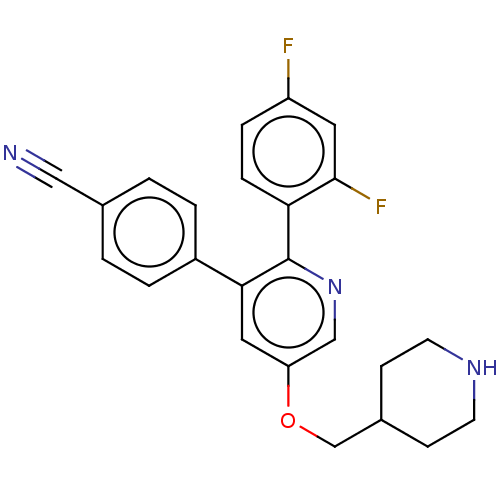
(CHEMBL3760085)Show SMILES Fc1ccc(c(F)c1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C24H21F2N3O/c25-19-5-6-21(23(26)11-19)24-22(18-3-1-16(13-27)2-4-18)12-20(14-29-24)30-15-17-7-9-28-10-8-17/h1-6,11-12,14,17,28H,7-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142186
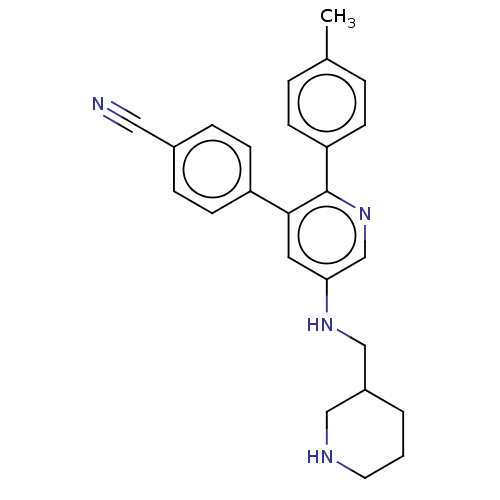
(CHEMBL3760091)Show SMILES Cc1ccc(cc1)-c1ncc(NCC2CCCNC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H26N4/c1-18-4-8-22(9-5-18)25-24(21-10-6-19(14-26)7-11-21)13-23(17-29-25)28-16-20-3-2-12-27-15-20/h4-11,13,17,20,27-28H,2-3,12,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400523
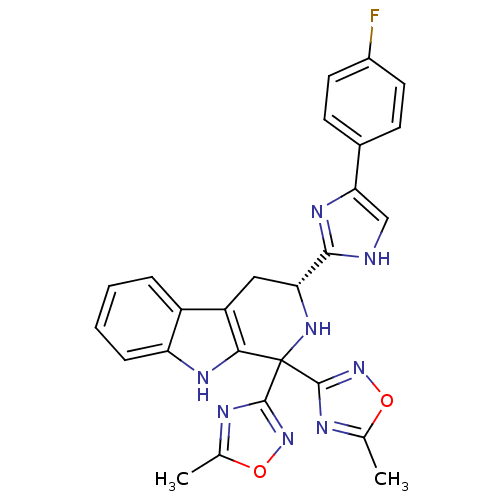
(CHEMBL2204940)Show SMILES Cc1nc(no1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1noc(C)n1 |r| Show InChI InChI=1S/C26H21FN8O2/c1-13-29-24(34-36-13)26(25-30-14(2)37-35-25)22-18(17-5-3-4-6-19(17)31-22)11-20(33-26)23-28-12-21(32-23)15-7-9-16(27)10-8-15/h3-10,12,20,31,33H,11H2,1-2H3,(H,28,32)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400527
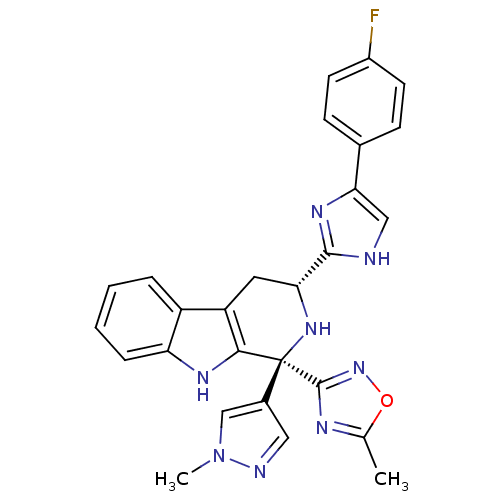
(CHEMBL2204936)Show SMILES Cc1nc(no1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data