 Found 1828 hits with Last Name = 'sherer' and Initial = 'e'
Found 1828 hits with Last Name = 'sherer' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126916
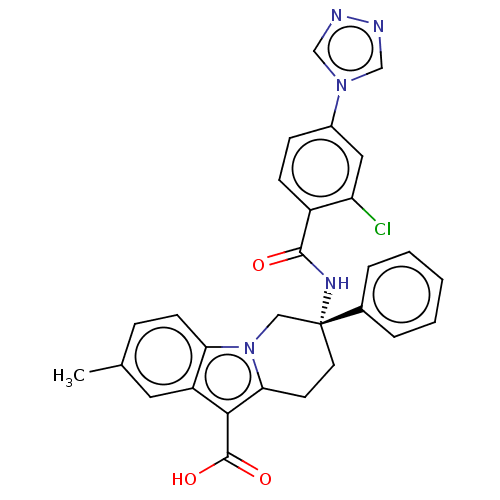
(CHEMBL3629111 | US10351558, Example 139)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(O)=O)c2c1)(NC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24ClN5O3/c1-18-7-10-24-22(13-18)26(28(37)38)25-11-12-29(15-35(24)25,19-5-3-2-4-6-19)33-27(36)21-9-8-20(14-23(21)30)34-16-31-32-17-34/h2-10,13-14,16-17H,11-12,15H2,1H3,(H,33,36)(H,37,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126919
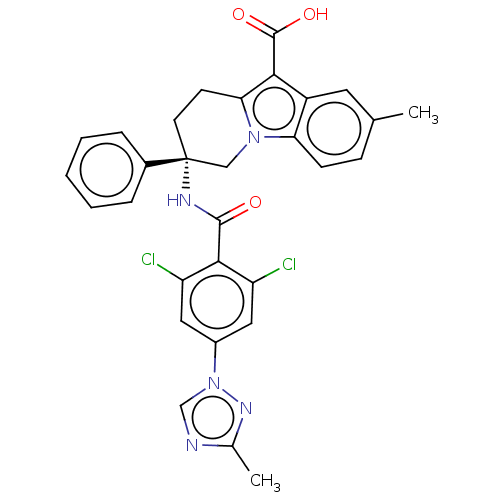
(CHEMBL3629114)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3c(C(O)=O)c4cc(C)ccc4n3C2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C30H25Cl2N5O3/c1-17-8-9-24-21(12-17)26(29(39)40)25-10-11-30(15-36(24)25,19-6-4-3-5-7-19)34-28(38)27-22(31)13-20(14-23(27)32)37-16-33-18(2)35-37/h3-9,12-14,16H,10-11,15H2,1-2H3,(H,34,38)(H,39,40)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50369890
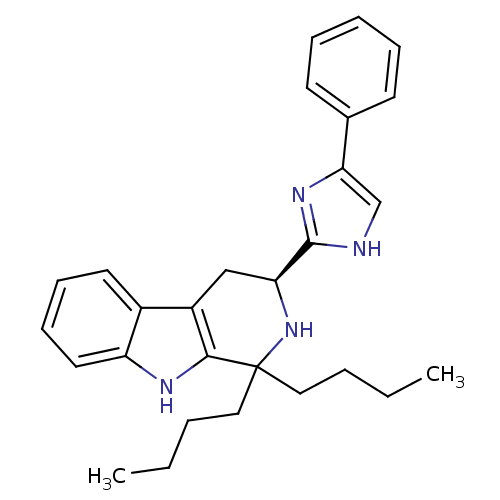
(CHEMBL1237140 | CHEMBL1788167)Show SMILES CCCCC1(CCCC)N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468037
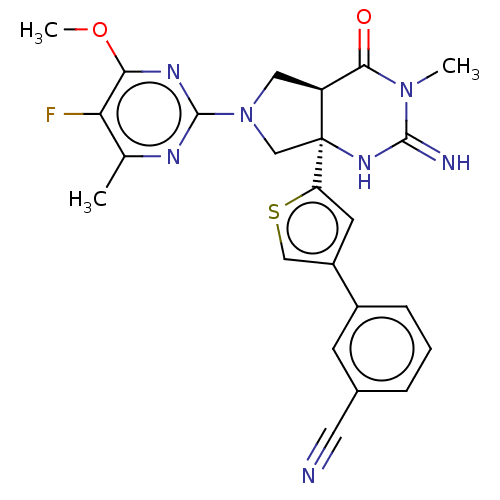
(CHEMBL4293298)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1nc(C)c(F)c(OC)n1 |r| Show InChI InChI=1S/C24H22FN7O2S/c1-13-19(25)20(34-3)29-23(28-13)32-10-17-21(33)31(2)22(27)30-24(17,12-32)18-8-16(11-35-18)15-6-4-5-14(7-15)9-26/h4-8,11,17H,10,12H2,1-3H3,(H2,27,30)/t17-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468040
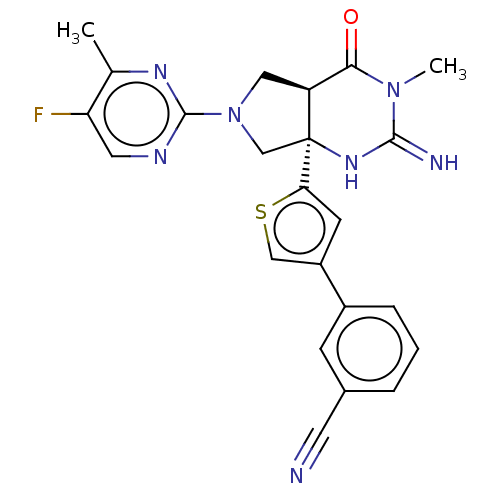
(CHEMBL4289763)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(C)n1 |r| Show InChI InChI=1S/C23H20FN7OS/c1-13-18(24)9-27-22(28-13)31-10-17-20(32)30(2)21(26)29-23(17,12-31)19-7-16(11-33-19)15-5-3-4-14(6-15)8-25/h3-7,9,11,17H,10,12H2,1-2H3,(H2,26,29)/t17-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126872
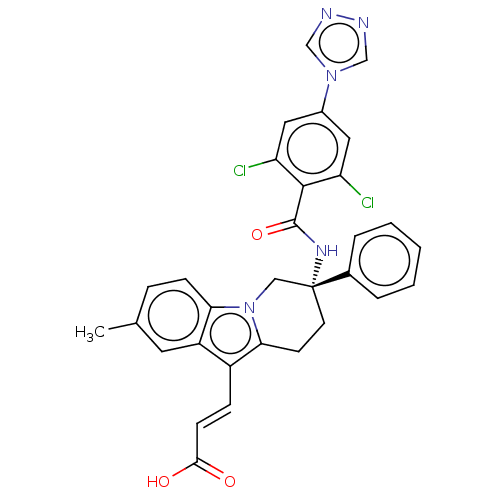
(CHEMBL3628964)Show SMILES Cc1ccc2n3C[C@@](CCc3c(\C=C\C(O)=O)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C31H25Cl2N5O3/c1-19-7-9-26-23(13-19)22(8-10-28(39)40)27-11-12-31(16-38(26)27,20-5-3-2-4-6-20)36-30(41)29-24(32)14-21(15-25(29)33)37-17-34-35-18-37/h2-10,13-15,17-18H,11-12,16H2,1H3,(H,36,41)(H,39,40)/b10-8+/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021074

(CHEMBL3287628)Show SMILES Clc1ccc(CN[C@H]2CCCC[C@H]2NC(=O)c2ccc3ccccc3c2)cc1Cl |r| Show InChI InChI=1S/C24H24Cl2N2O/c25-20-12-9-16(13-21(20)26)15-27-22-7-3-4-8-23(22)28-24(29)19-11-10-17-5-1-2-6-18(17)14-19/h1-2,5-6,9-14,22-23,27H,3-4,7-8,15H2,(H,28,29)/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468038
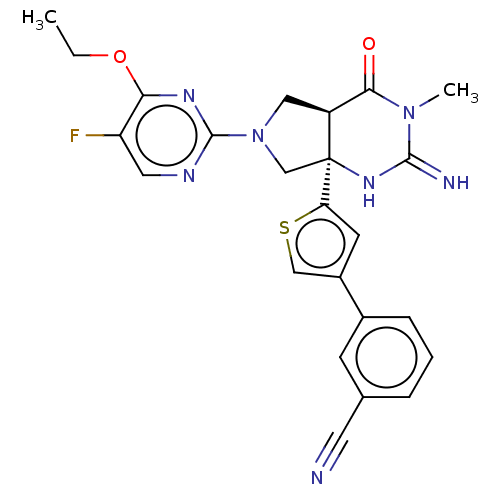
(CHEMBL4294236)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OCC)n1 |r| Show InChI InChI=1S/C24H22FN7O2S/c1-3-34-20-18(25)10-28-23(29-20)32-11-17-21(33)31(2)22(27)30-24(17,13-32)19-8-16(12-35-19)15-6-4-5-14(7-15)9-26/h4-8,10,12,17H,3,11,13H2,1-2H3,(H2,27,30)/t17-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468047

(CHEMBL4278011)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OCCC)n1 |r| Show InChI InChI=1S/C25H24FN7O2S/c1-3-7-35-21-19(26)11-29-24(30-21)33-12-18-22(34)32(2)23(28)31-25(18,14-33)20-9-17(13-36-20)16-6-4-5-15(8-16)10-27/h4-6,8-9,11,13,18H,3,7,12,14H2,1-2H3,(H2,28,31)/t18-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468046

(CHEMBL4278154)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(n1)N1CCOCC1 |r| Show InChI InChI=1S/C26H25FN8O2S/c1-33-23(36)19-13-35(25-30-12-20(27)22(31-25)34-5-7-37-8-6-34)15-26(19,32-24(33)29)21-10-18(14-38-21)17-4-2-3-16(9-17)11-28/h2-4,9-10,12,14,19H,5-8,13,15H2,1H3,(H2,29,32)/t19-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468043

(CHEMBL4288838)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1nc(C)c(F)c(C)n1 |r| Show InChI InChI=1S/C24H22FN7OS/c1-13-20(25)14(2)29-23(28-13)32-10-18-21(33)31(3)22(27)30-24(18,12-32)19-8-17(11-34-19)16-6-4-5-15(7-16)9-26/h4-8,11,18H,10,12H2,1-3H3,(H2,27,30)/t18-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468048

(CHEMBL4280271)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(Cl)c1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C21H18ClFN6OS/c1-28-18(30)16-9-29(20-25-7-15(23)8-26-20)11-21(16,27-19(28)24)17-6-13(10-31-17)12-3-2-4-14(22)5-12/h2-8,10,16H,9,11H2,1H3,(H2,24,27)/t16-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50164512
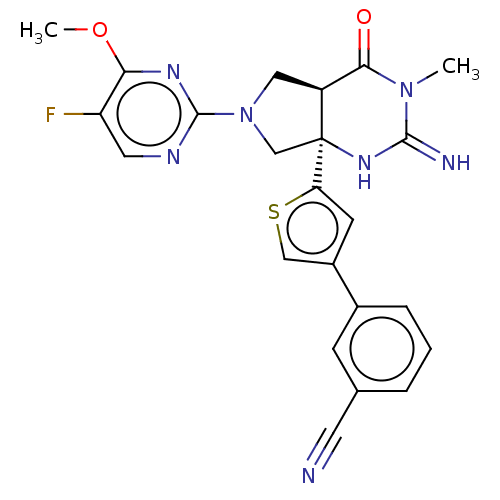
(CHEMBL3800286)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OC)n1 |r| Show InChI InChI=1S/C23H20FN7O2S/c1-30-20(32)16-10-31(22-27-9-17(24)19(28-22)33-2)12-23(16,29-21(30)26)18-7-15(11-34-18)14-5-3-4-13(6-14)8-25/h3-7,9,11,16H,10,12H2,1-2H3,(H2,26,29)/t16-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125979
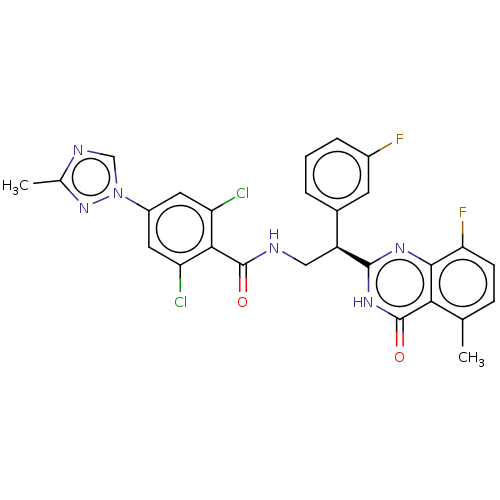
(CHEMBL3627899)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2nc3c(F)ccc(C)c3c(=O)[nH]2)c(Cl)c1 |r| Show InChI InChI=1S/C27H20Cl2F2N6O2/c1-13-6-7-21(31)24-22(13)27(39)35-25(34-24)18(15-4-3-5-16(30)8-15)11-32-26(38)23-19(28)9-17(10-20(23)29)37-12-33-14(2)36-37/h3-10,12,18H,11H2,1-2H3,(H,32,38)(H,34,35,39)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468039

(CHEMBL4278329)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cc(OC)cc(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H20FN7O2S/c1-30-20(32)18-10-31(22-27-8-16(24)9-28-22)12-23(18,29-21(30)26)19-6-15(11-34-19)14-3-13(7-25)4-17(5-14)33-2/h3-6,8-9,11,18H,10,12H2,1-2H3,(H2,26,29)/t18-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468044

(CHEMBL4279084)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(CC)n1 |r| Show InChI InChI=1S/C24H22FN7OS/c1-3-19-18(25)10-28-23(29-19)32-11-17-21(33)31(2)22(27)30-24(17,13-32)20-8-16(12-34-20)15-6-4-5-14(7-15)9-26/h4-8,10,12,17H,3,11,13H2,1-2H3,(H2,27,30)/t17-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126956
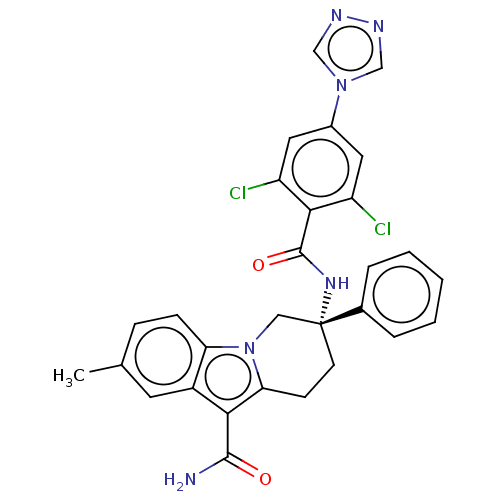
(CHEMBL3628961 | US10351558, Example 174)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(N)=O)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24Cl2N6O2/c1-17-7-8-23-20(11-17)25(27(32)38)24-9-10-29(14-37(23)24,18-5-3-2-4-6-18)35-28(39)26-21(30)12-19(13-22(26)31)36-15-33-34-16-36/h2-8,11-13,15-16H,9-10,14H2,1H3,(H2,32,38)(H,35,39)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110470

(CHEMBL3605798)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCCCc1ccccc1 Show InChI InChI=1S/C24H29N3/c1-19-11-13-21(14-12-19)22-18-25-23(27-22)24(15-5-6-16-24)26-17-7-10-20-8-3-2-4-9-20/h2-4,8-9,11-14,18,26H,5-7,10,15-17H2,1H3,(H,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126913
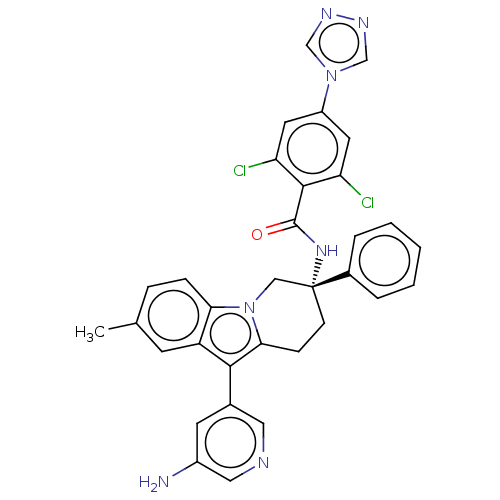
(CHEMBL3628966)Show SMILES Cc1ccc2n3C[C@@](CCc3c(-c3cncc(N)c3)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C33H27Cl2N7O/c1-20-7-8-28-25(11-20)30(21-12-23(36)16-37-15-21)29-9-10-33(17-42(28)29,22-5-3-2-4-6-22)40-32(43)31-26(34)13-24(14-27(31)35)41-18-38-39-19-41/h2-8,11-16,18-19H,9-10,17,36H2,1H3,(H,40,43)/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126949
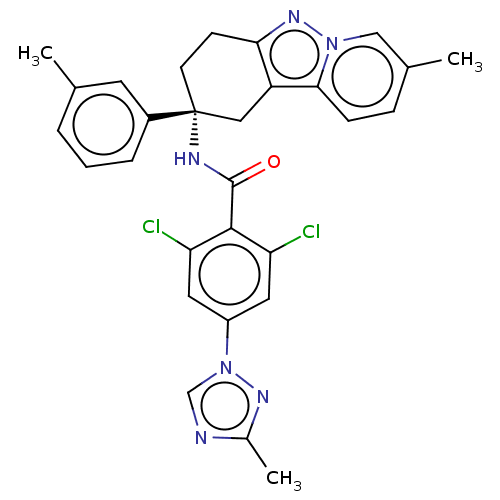
(CHEMBL3628954)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2cccc(C)c2)c(Cl)c1 |r| Show InChI InChI=1S/C29H26Cl2N6O/c1-17-5-4-6-20(11-17)29(10-9-25-22(14-29)26-8-7-18(2)15-36(26)35-25)33-28(38)27-23(30)12-21(13-24(27)31)37-16-32-19(3)34-37/h4-8,11-13,15-16H,9-10,14H2,1-3H3,(H,33,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110461

(CHEMBL3605789)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCc1c[nH]c2ccccc12 Show InChI InChI=1S/C23H23FN4/c24-18-9-7-16(8-10-18)21-15-26-22(28-21)23(11-3-4-12-23)27-14-17-13-25-20-6-2-1-5-19(17)20/h1-2,5-10,13,15,25,27H,3-4,11-12,14H2,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468045

(CHEMBL4288644)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cc(F)cc(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H17F2N7OS/c1-30-19(32)17-9-31(21-27-7-16(24)8-28-21)11-22(17,29-20(30)26)18-5-14(10-33-18)13-2-12(6-25)3-15(23)4-13/h2-5,7-8,10,17H,9,11H2,1H3,(H2,26,29)/t17-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126941
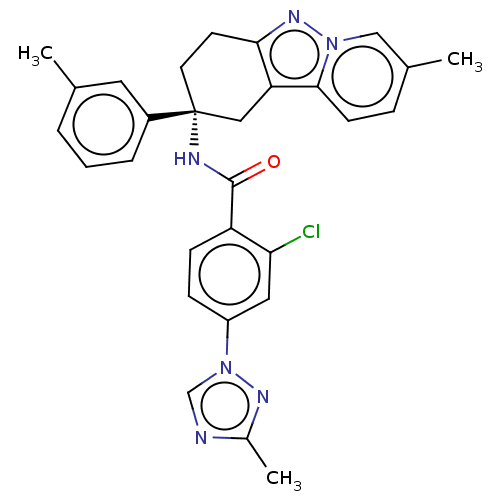
(CHEMBL3628838)Show SMILES Cc1ncn(n1)-c1ccc(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2cccc(C)c2)c(Cl)c1 |r| Show InChI InChI=1S/C29H27ClN6O/c1-18-5-4-6-21(13-18)29(12-11-26-24(15-29)27-10-7-19(2)16-35(27)34-26)32-28(37)23-9-8-22(14-25(23)30)36-17-31-20(3)33-36/h4-10,13-14,16-17H,11-12,15H2,1-3H3,(H,32,37)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126912
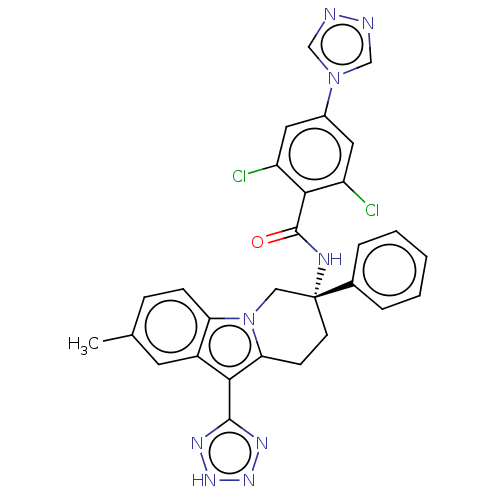
(CHEMBL3628965)Show SMILES Cc1ccc2n3C[C@@](CCc3c(-c3nn[nH]n3)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H23Cl2N9O/c1-17-7-8-23-20(11-17)25(27-35-37-38-36-27)24-9-10-29(14-40(23)24,18-5-3-2-4-6-18)34-28(41)26-21(30)12-19(13-22(26)31)39-15-32-33-16-39/h2-8,11-13,15-16H,9-10,14H2,1H3,(H,34,41)(H,35,36,37,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126855
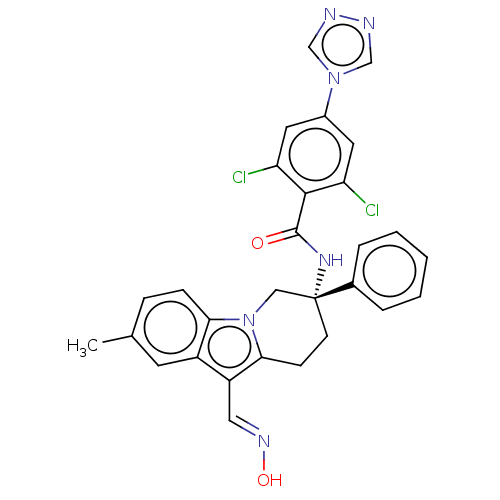
(CHEMBL3628962 | US10351558, Example 173)Show SMILES Cc1ccc2n3C[C@@](CCc3c(\C=N\O)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24Cl2N6O2/c1-18-7-8-25-21(11-18)22(14-34-39)26-9-10-29(15-37(25)26,19-5-3-2-4-6-19)35-28(38)27-23(30)12-20(13-24(27)31)36-16-32-33-17-36/h2-8,11-14,16-17,39H,9-10,15H2,1H3,(H,35,38)/b34-14+/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126917
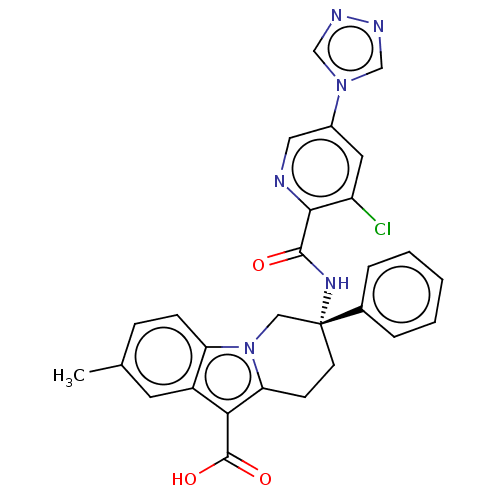
(CHEMBL3629112)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(O)=O)c2c1)(NC(=O)c1ncc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C28H23ClN6O3/c1-17-7-8-22-20(11-17)24(27(37)38)23-9-10-28(14-35(22)23,18-5-3-2-4-6-18)33-26(36)25-21(29)12-19(13-30-25)34-15-31-32-16-34/h2-8,11-13,15-16H,9-10,14H2,1H3,(H,33,36)(H,37,38)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126915
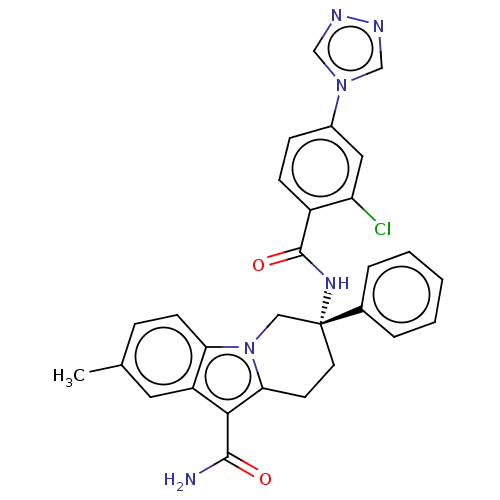
(CHEMBL3629110 | US10351558, Example 138)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C(N)=O)c2c1)(NC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H25ClN6O2/c1-18-7-10-24-22(13-18)26(27(31)37)25-11-12-29(15-36(24)25,19-5-3-2-4-6-19)34-28(38)21-9-8-20(14-23(21)30)35-16-32-33-17-35/h2-10,13-14,16-17H,11-12,15H2,1H3,(H2,31,37)(H,34,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126926
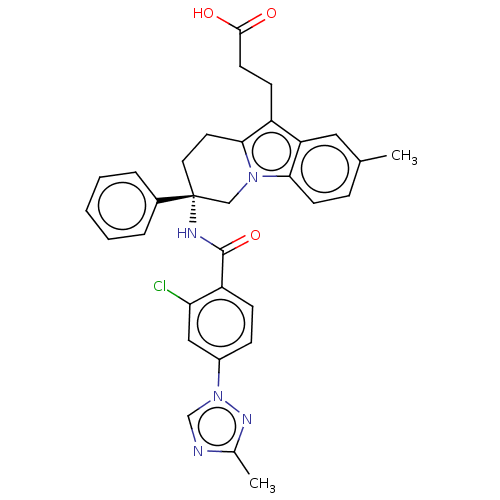
(CHEMBL3628835 | US10351558, Example 183)Show SMILES Cc1ncn(n1)-c1ccc(C(=O)N[C@@]2(CCc3c(CCC(O)=O)c4cc(C)ccc4n3C2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C32H30ClN5O3/c1-20-8-12-28-26(16-20)24(11-13-30(39)40)29-14-15-32(18-37(28)29,22-6-4-3-5-7-22)35-31(41)25-10-9-23(17-27(25)33)38-19-34-21(2)36-38/h3-10,12,16-17,19H,11,13-15,18H2,1-2H3,(H,35,41)(H,39,40)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126856
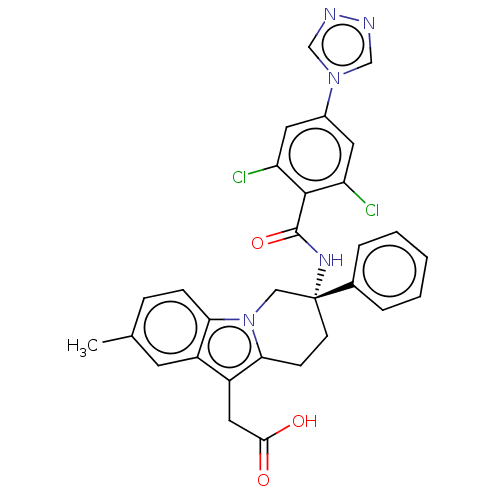
(CHEMBL3628963)Show SMILES Cc1ccc2n3C[C@@](CCc3c(CC(O)=O)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C30H25Cl2N5O3/c1-18-7-8-25-21(11-18)22(14-27(38)39)26-9-10-30(15-37(25)26,19-5-3-2-4-6-19)35-29(40)28-23(31)12-20(13-24(28)32)36-16-33-34-17-36/h2-8,11-13,16-17H,9-10,14-15H2,1H3,(H,35,40)(H,38,39)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125972
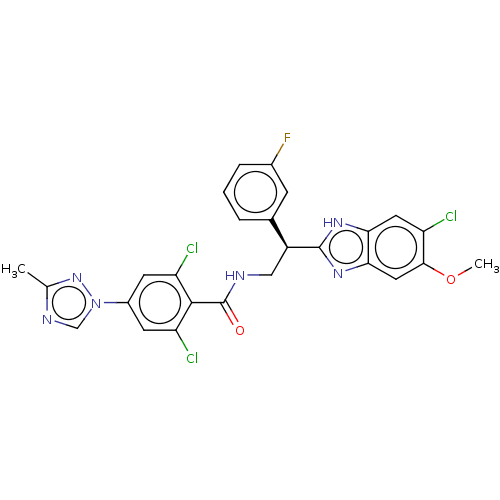
(CHEMBL3627894)Show SMILES COc1cc2nc([nH]c2cc1Cl)[C@@H](CNC(=O)c1c(Cl)cc(cc1Cl)-n1cnc(C)n1)c1cccc(F)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O2/c1-13-32-12-36(35-13)16-7-19(28)24(20(29)8-16)26(37)31-11-17(14-4-3-5-15(30)6-14)25-33-21-9-18(27)23(38-2)10-22(21)34-25/h3-10,12,17H,11H2,1-2H3,(H,31,37)(H,33,34)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126942
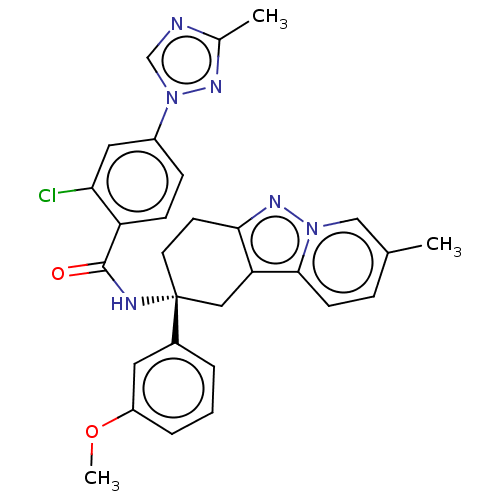
(CHEMBL3628947)Show SMILES COc1cccc(c1)[C@]1(CCc2nn3cc(C)ccc3c2C1)NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1 |r| Show InChI InChI=1S/C29H27ClN6O2/c1-18-7-10-27-24-15-29(12-11-26(24)34-35(27)16-18,20-5-4-6-22(13-20)38-3)32-28(37)23-9-8-21(14-25(23)30)36-17-31-19(2)33-36/h4-10,13-14,16-17H,11-12,15H2,1-3H3,(H,32,37)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021075
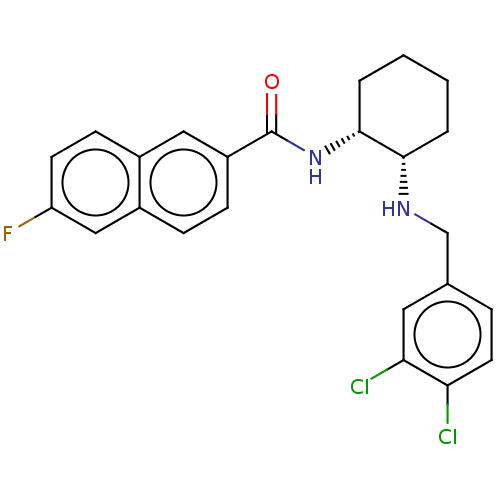
(CHEMBL3287629)Show SMILES Fc1ccc2cc(ccc2c1)C(=O)N[C@@H]1CCCC[C@@H]1NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C24H23Cl2FN2O/c25-20-10-5-15(11-21(20)26)14-28-22-3-1-2-4-23(22)29-24(30)18-7-6-17-13-19(27)9-8-16(17)12-18/h5-13,22-23,28H,1-4,14H2,(H,29,30)/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468035
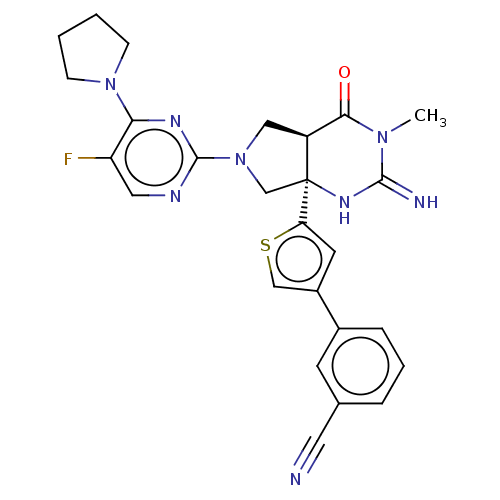
(CHEMBL4285940)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(n1)N1CCCC1 |r| Show InChI InChI=1S/C26H25FN8OS/c1-33-23(36)19-13-35(25-30-12-20(27)22(31-25)34-7-2-3-8-34)15-26(19,32-24(33)29)21-10-18(14-37-21)17-6-4-5-16(9-17)11-28/h4-6,9-10,12,14,19H,2-3,7-8,13,15H2,1H3,(H2,29,32)/t19-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126944
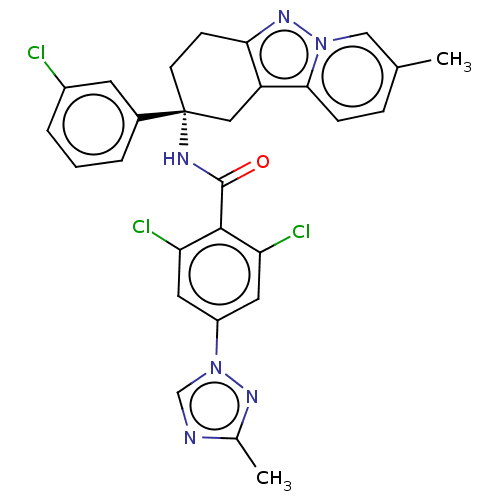
(CHEMBL3628949)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2cccc(Cl)c2)c(Cl)c1 |r| Show InChI InChI=1S/C28H23Cl3N6O/c1-16-6-7-25-21-13-28(18-4-3-5-19(29)10-18,9-8-24(21)35-36(25)14-16)33-27(38)26-22(30)11-20(12-23(26)31)37-15-32-17(2)34-37/h3-7,10-12,14-15H,8-9,13H2,1-2H3,(H,33,38)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125980
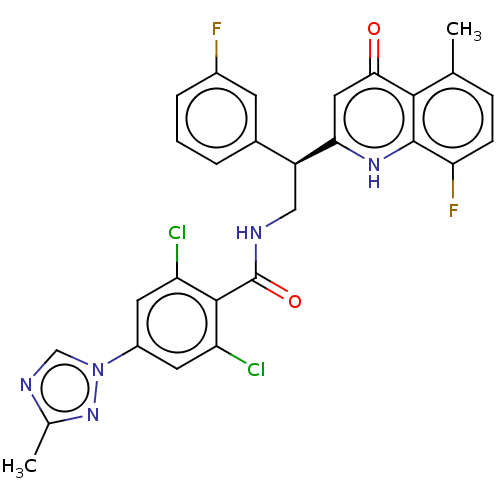
(CHEMBL3627900)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cc(=O)c3c(C)ccc(F)c3[nH]2)c(Cl)c1 |r| Show InChI InChI=1S/C28H21Cl2F2N5O2/c1-14-6-7-22(32)27-25(14)24(38)11-23(35-27)19(16-4-3-5-17(31)8-16)12-33-28(39)26-20(29)9-18(10-21(26)30)37-13-34-15(2)36-37/h3-11,13,19H,12H2,1-2H3,(H,33,39)(H,35,38)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110440
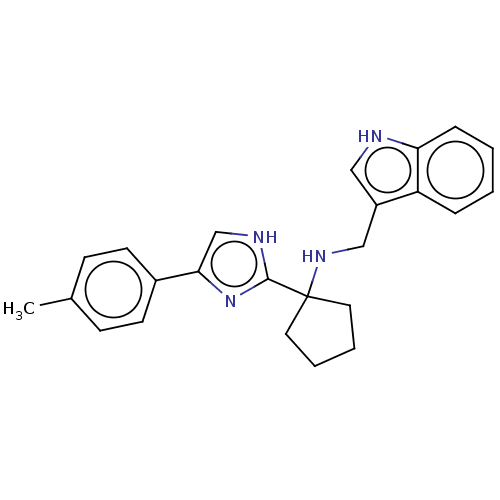
(CHEMBL3605785)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCc1c[nH]c2ccccc12 Show InChI InChI=1S/C24H26N4/c1-17-8-10-18(11-9-17)22-16-26-23(28-22)24(12-4-5-13-24)27-15-19-14-25-21-7-3-2-6-20(19)21/h2-3,6-11,14,16,25,27H,4-5,12-13,15H2,1H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021090
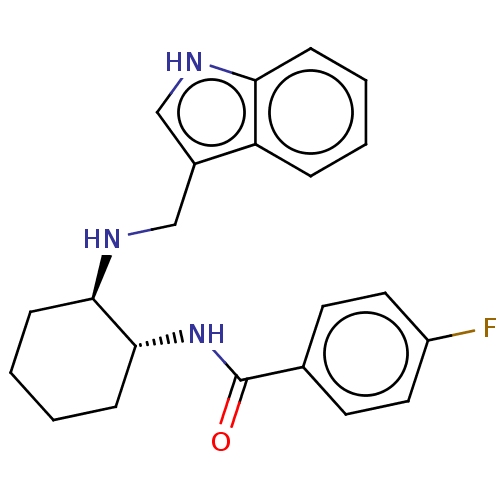
(CHEMBL3287632)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCCC[C@H]1NCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C22H24FN3O/c23-17-11-9-15(10-12-17)22(27)26-21-8-4-3-7-20(21)25-14-16-13-24-19-6-2-1-5-18(16)19/h1-2,5-6,9-13,20-21,24-25H,3-4,7-8,14H2,(H,26,27)/t20-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125978
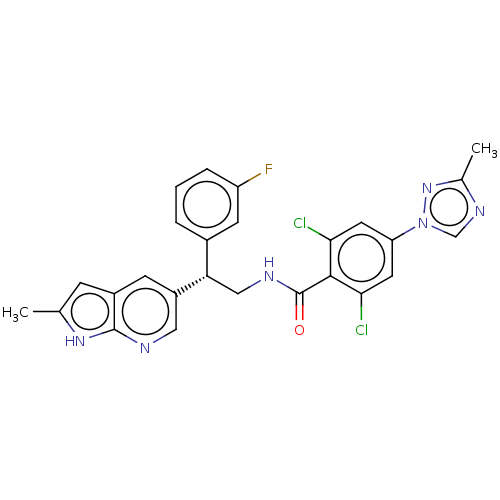
(CHEMBL3627898 | US10189819, Example 77)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cnc3[nH]c(C)cc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-6-17-7-18(11-30-25(17)33-14)21(16-4-3-5-19(29)8-16)12-31-26(36)24-22(27)9-20(10-23(24)28)35-13-32-15(2)34-35/h3-11,13,21H,12H2,1-2H3,(H,30,33)(H,31,36)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021063
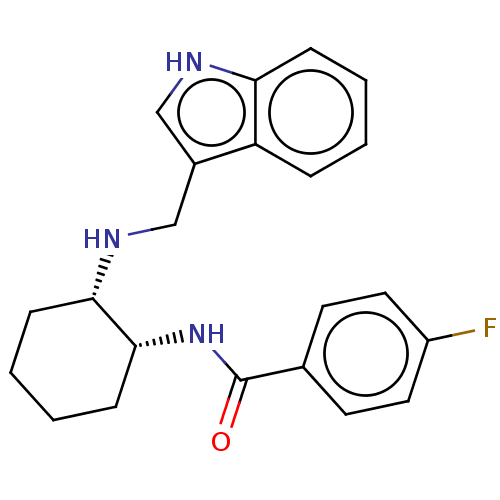
(CHEMBL3287613)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCCC[C@@H]1NCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C22H24FN3O/c23-17-11-9-15(10-12-17)22(27)26-21-8-4-3-7-20(21)25-14-16-13-24-19-6-2-1-5-18(16)19/h1-2,5-6,9-13,20-21,24-25H,3-4,7-8,14H2,(H,26,27)/t20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468036

(CHEMBL4286732)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1ccc(F)c(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H17F2N7OS/c1-30-19(32)16-9-31(21-27-7-15(23)8-28-21)11-22(16,29-20(30)26)18-5-14(10-33-18)12-2-3-17(24)13(4-12)6-25/h2-5,7-8,10,16H,9,11H2,1H3,(H2,26,29)/t16-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398693
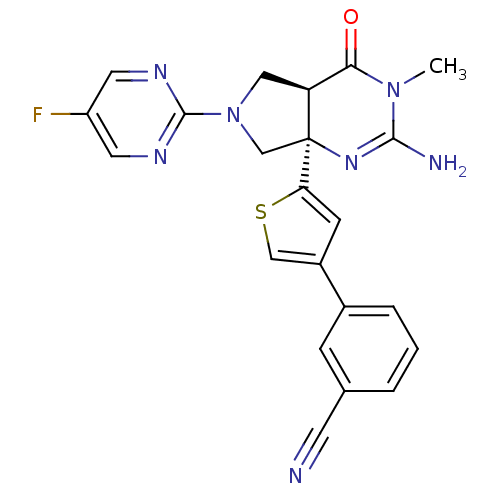
(CHEMBL2178718)Show SMILES CN1C(N)=N[C@]2(CN(C[C@H]2C1=O)c1ncc(F)cn1)c1cc(cs1)-c1cccc(c1)C#N |r,c:3| Show InChI InChI=1S/C22H18FN7OS/c1-29-19(31)17-10-30(21-26-8-16(23)9-27-21)12-22(17,28-20(29)25)18-6-15(11-32-18)14-4-2-3-13(5-14)7-24/h2-6,8-9,11,17H,10,12H2,1H3,(H2,25,28)/t17-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021109
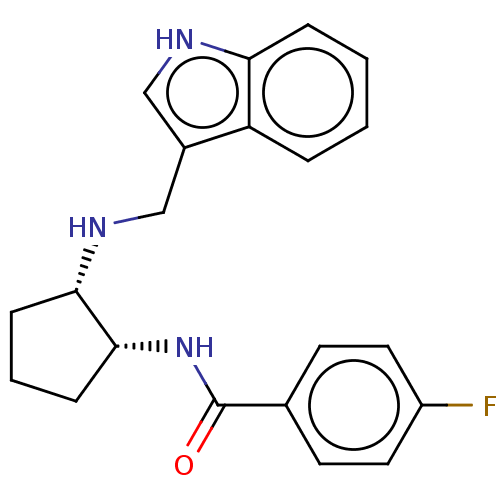
(CHEMBL3287633)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCC[C@@H]1NCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C21H22FN3O/c22-16-10-8-14(9-11-16)21(26)25-20-7-3-6-19(20)24-13-15-12-23-18-5-2-1-4-17(15)18/h1-2,4-5,8-12,19-20,23-24H,3,6-7,13H2,(H,25,26)/t19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126946
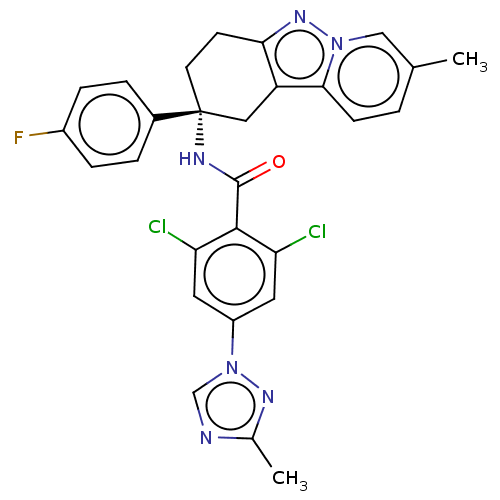
(CHEMBL3628951)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2ccc(F)cc2)c(Cl)c1 |r| Show InChI InChI=1S/C28H23Cl2FN6O/c1-16-3-8-25-21-13-28(18-4-6-19(31)7-5-18,10-9-24(21)35-36(25)14-16)33-27(38)26-22(29)11-20(12-23(26)30)37-15-32-17(2)34-37/h3-8,11-12,14-15H,9-10,13H2,1-2H3,(H,33,38)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126945
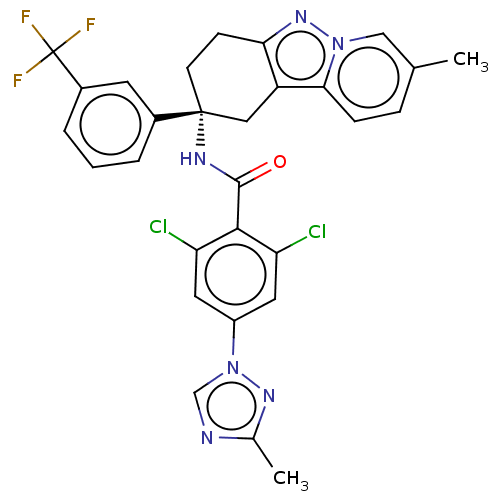
(CHEMBL3628950)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)N[C@@]2(CCc3nn4cc(C)ccc4c3C2)c2cccc(c2)C(F)(F)F)c(Cl)c1 |r| Show InChI InChI=1S/C29H23Cl2F3N6O/c1-16-6-7-25-21-13-28(9-8-24(21)38-39(25)14-16,18-4-3-5-19(10-18)29(32,33)34)36-27(41)26-22(30)11-20(12-23(26)31)40-15-35-17(2)37-40/h3-7,10-12,14-15H,8-9,13H2,1-2H3,(H,36,41)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021073
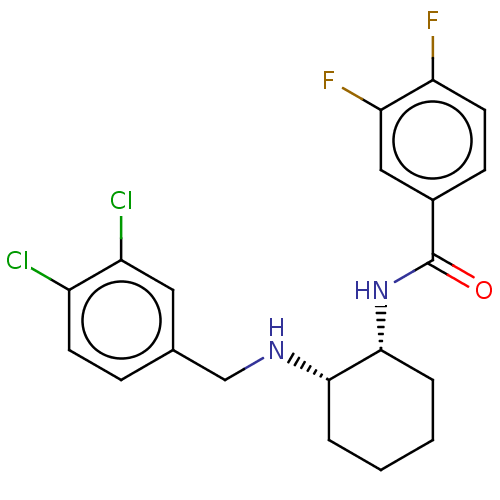
(CHEMBL3287627)Show SMILES Fc1ccc(cc1F)C(=O)N[C@@H]1CCCC[C@@H]1NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C20H20Cl2F2N2O/c21-14-7-5-12(9-15(14)22)11-25-18-3-1-2-4-19(18)26-20(27)13-6-8-16(23)17(24)10-13/h5-10,18-19,25H,1-4,11H2,(H,26,27)/t18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110467

(CHEMBL3605795)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCc1cc2ccccc2[nH]1 Show InChI InChI=1S/C24H26N4/c1-17-8-10-18(11-9-17)22-16-25-23(28-22)24(12-4-5-13-24)26-15-20-14-19-6-2-3-7-21(19)27-20/h2-3,6-11,14,16,26-27H,4-5,12-13,15H2,1H3,(H,25,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126948
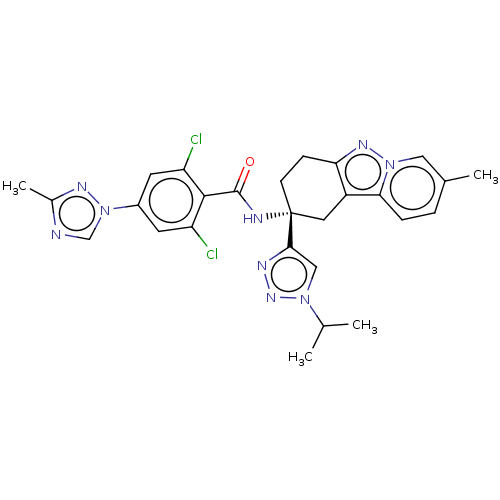
(CHEMBL3628953)Show SMILES CC(C)n1cc(nn1)[C@]1(CCc2nn3cc(C)ccc3c2C1)NC(=O)c1c(Cl)cc(cc1Cl)-n1cnc(C)n1 |r| Show InChI InChI=1S/C27H27Cl2N9O/c1-15(2)36-13-24(32-35-36)27(8-7-22-19(11-27)23-6-5-16(3)12-37(23)34-22)31-26(39)25-20(28)9-18(10-21(25)29)38-14-30-17(4)33-38/h5-6,9-10,12-15H,7-8,11H2,1-4H3,(H,31,39)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50126955
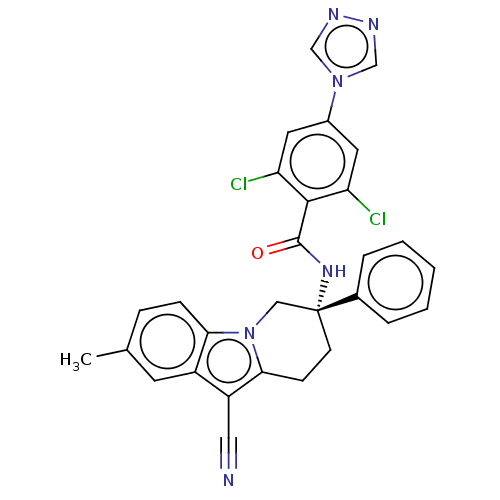
(CHEMBL3628960 | US10351558, Example 152)Show SMILES Cc1ccc2n3C[C@@](CCc3c(C#N)c2c1)(NC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C29H22Cl2N6O/c1-18-7-8-25-21(11-18)22(14-32)26-9-10-29(15-37(25)26,19-5-3-2-4-6-19)35-28(38)27-23(30)12-20(13-24(27)31)36-16-33-34-17-36/h2-8,11-13,16-17H,9-10,15H2,1H3,(H,35,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 5437-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.078
BindingDB Entry DOI: 10.7270/Q2B859X6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110441
(CHEMBL3605786)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCCC1)NCc1c[nH]c2ccccc12 Show InChI InChI=1S/C25H28N4/c1-18-9-11-19(12-10-18)23-17-27-24(29-23)25(13-5-2-6-14-25)28-16-20-15-26-22-8-4-3-7-21(20)22/h3-4,7-12,15,17,26,28H,2,5-6,13-14,16H2,1H3,(H,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110460

(CHEMBL3605788)Show SMILES C(NC1(CCCC1)c1nc(c[nH]1)-c1ccccc1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C23H24N4/c1-2-8-17(9-3-1)21-16-25-22(27-21)23(12-6-7-13-23)26-15-18-14-24-20-11-5-4-10-19(18)20/h1-5,8-11,14,16,24,26H,6-7,12-13,15H2,(H,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data