| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50134771 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_1549435 |
|---|
| IC50 | 3000±n/a nM |
|---|
| Citation |  Ammirati, M; Bagley, SW; Bhattacharya, SK; Buckbinder, L; Carlo, AA; Conrad, R; Cortes, C; Dow, RL; Dowling, MS; El-Kattan, A; Ford, K; Guimar„es, CR; Hepworth, D; Jiao, W; LaPerle, J; Liu, S; Londregan, A; Loria, PM; Mathiowetz, AM; Munchhof, M; Orr, ST; Petersen, DN; Price, DA; Skoura, A; Smith, AC; Wang, J Discovery of an in Vivo Tool to Establish Proof-of-Concept for MAP4K4-Based Antidiabetic Treatment. ACS Med Chem Lett6:1128-33 (2015) [PubMed] Article Ammirati, M; Bagley, SW; Bhattacharya, SK; Buckbinder, L; Carlo, AA; Conrad, R; Cortes, C; Dow, RL; Dowling, MS; El-Kattan, A; Ford, K; Guimar„es, CR; Hepworth, D; Jiao, W; LaPerle, J; Liu, S; Londregan, A; Loria, PM; Mathiowetz, AM; Munchhof, M; Orr, ST; Petersen, DN; Price, DA; Skoura, A; Smith, AC; Wang, J Discovery of an in Vivo Tool to Establish Proof-of-Concept for MAP4K4-Based Antidiabetic Treatment. ACS Med Chem Lett6:1128-33 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50134771 |
|---|
| n/a |
|---|
| Name | BDBM50134771 |
|---|
| Synonyms: | CHEMBL3754515 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H13ClN4 |
|---|
| Mol. Mass. | 296.754 |
|---|
| SMILES | Nc1ccc(cn1)-c1cnc(N)c(c1)-c1ccc(Cl)cc1 |
|---|
| Structure | 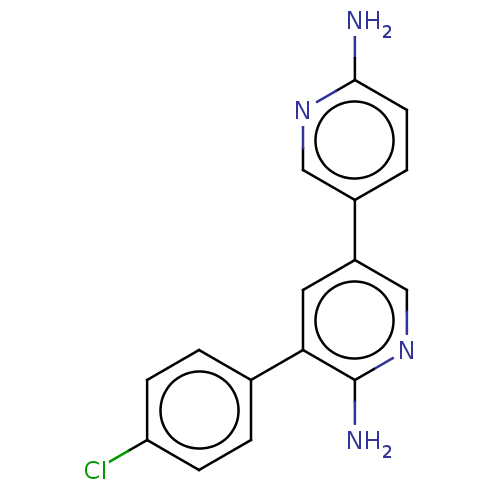
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ammirati, M; Bagley, SW; Bhattacharya, SK; Buckbinder, L; Carlo, AA; Conrad, R; Cortes, C; Dow, RL; Dowling, MS; El-Kattan, A; Ford, K; Guimar„es, CR; Hepworth, D; Jiao, W; LaPerle, J; Liu, S; Londregan, A; Loria, PM; Mathiowetz, AM; Munchhof, M; Orr, ST; Petersen, DN; Price, DA; Skoura, A; Smith, AC; Wang, J Discovery of an in Vivo Tool to Establish Proof-of-Concept for MAP4K4-Based Antidiabetic Treatment. ACS Med Chem Lett6:1128-33 (2015) [PubMed] Article
Ammirati, M; Bagley, SW; Bhattacharya, SK; Buckbinder, L; Carlo, AA; Conrad, R; Cortes, C; Dow, RL; Dowling, MS; El-Kattan, A; Ford, K; Guimar„es, CR; Hepworth, D; Jiao, W; LaPerle, J; Liu, S; Londregan, A; Loria, PM; Mathiowetz, AM; Munchhof, M; Orr, ST; Petersen, DN; Price, DA; Skoura, A; Smith, AC; Wang, J Discovery of an in Vivo Tool to Establish Proof-of-Concept for MAP4K4-Based Antidiabetic Treatment. ACS Med Chem Lett6:1128-33 (2015) [PubMed] Article