 Found 2463 hits with Last Name = 'hepworth' and Initial = 'd'
Found 2463 hits with Last Name = 'hepworth' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061718
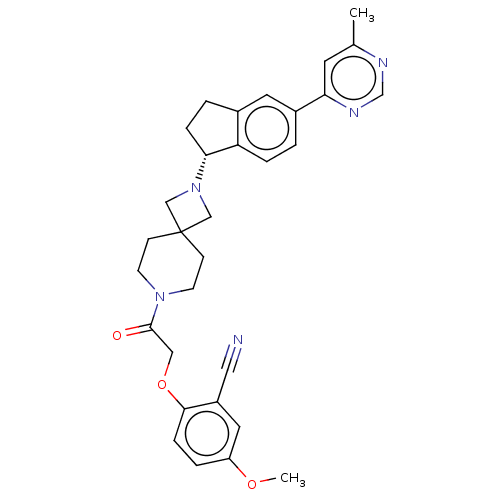
(CHEMBL3394200)Show SMILES COc1ccc(OCC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)c(c1)C#N |r| Show InChI InChI=1S/C31H33N5O3/c1-21-13-27(34-20-33-21)23-3-6-26-22(14-23)4-7-28(26)36-18-31(19-36)9-11-35(12-10-31)30(37)17-39-29-8-5-25(38-2)15-24(29)16-32/h3,5-6,8,13-15,20,28H,4,7,9-12,17-19H2,1-2H3/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019921
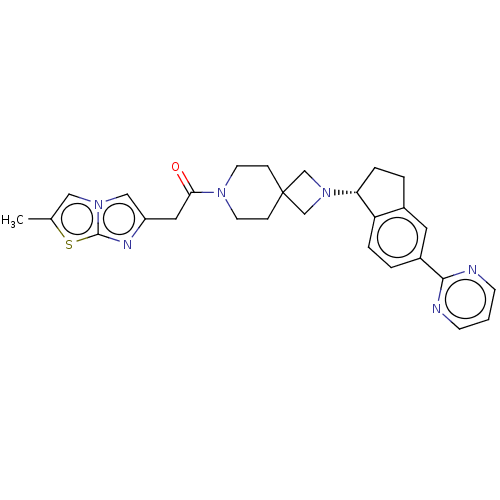
(CHEMBL3287213)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4ncccn4)CC3)nc2s1 |r| Show InChI InChI=1S/C28H30N6OS/c1-19-15-33-16-22(31-27(33)36-19)14-25(35)32-11-7-28(8-12-32)17-34(18-28)24-6-4-20-13-21(3-5-23(20)24)26-29-9-2-10-30-26/h2-3,5,9-10,13,15-16,24H,4,6-8,11-12,14,17-18H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315675
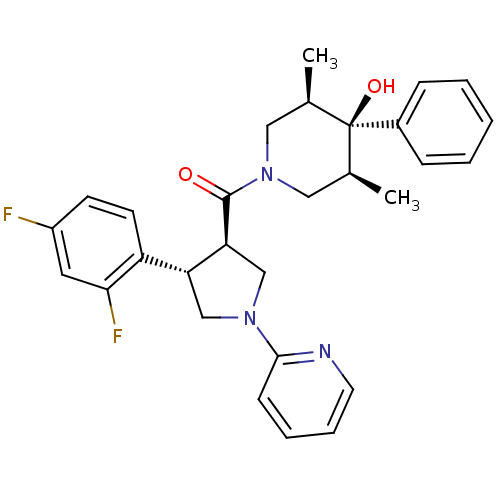
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-2-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1ccccn1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-8-4-3-5-9-21)28(35)25-18-33(27-10-6-7-13-32-27)17-24(25)23-12-11-22(30)14-26(23)31/h3-14,19-20,24-25,36H,15-18H2,1-2H3/t19-,20+,24-,25+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315688
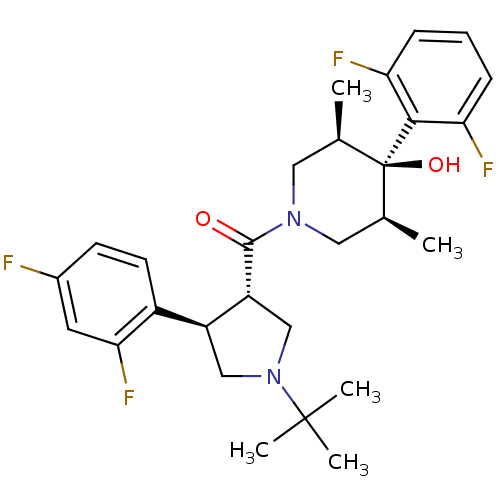
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1c(F)cccc1F)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H34F4N2O2/c1-16-12-33(13-17(2)28(16,36)25-22(30)7-6-8-23(25)31)26(35)21-15-34(27(3,4)5)14-20(21)19-10-9-18(29)11-24(19)32/h6-11,16-17,20-21,36H,12-15H2,1-5H3/t16-,17+,20-,21+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315674
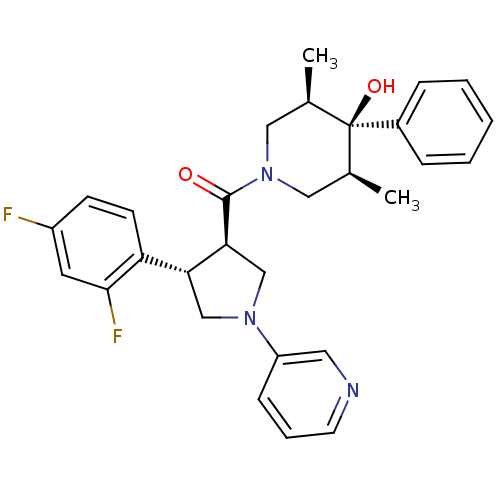
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-3-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnc1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-7-4-3-5-8-21)28(35)26-18-33(23-9-6-12-32-14-23)17-25(26)24-11-10-22(30)13-27(24)31/h3-14,19-20,25-26,36H,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315676
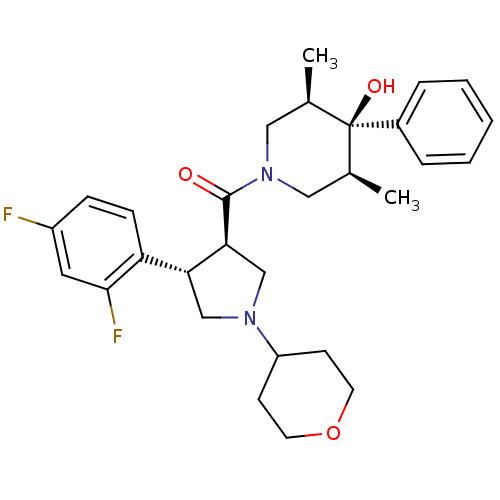
(((3R,4S)-4-(2,4-difluorophenyl)-1-(tetrahydro-2H-p...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCOCC1 |r| Show InChI InChI=1S/C29H36F2N2O3/c1-19-15-33(16-20(2)29(19,35)21-6-4-3-5-7-21)28(34)26-18-32(23-10-12-36-13-11-23)17-25(26)24-9-8-22(30)14-27(24)31/h3-9,14,19-20,23,25-26,35H,10-13,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315686
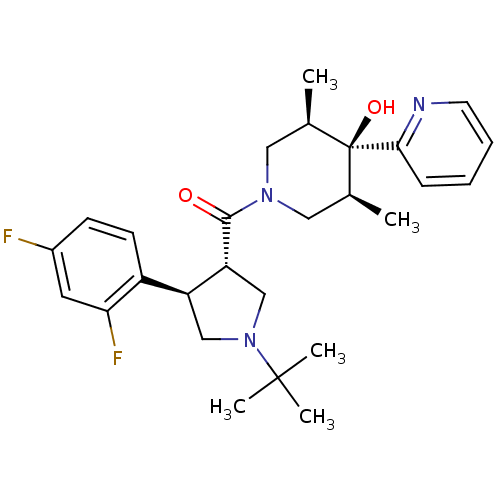
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccn1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C27H35F2N3O2/c1-17-13-31(14-18(2)27(17,34)24-8-6-7-11-30-24)25(33)22-16-32(26(3,4)5)15-21(22)20-10-9-19(28)12-23(20)29/h6-12,17-18,21-22,34H,13-16H2,1-5H3/t17-,18+,21-,22+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315677

(((3R,4S)-1-cyclobutyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCC1 |r| Show InChI InChI=1S/C28H34F2N2O2/c1-18-14-32(15-19(2)28(18,34)20-7-4-3-5-8-20)27(33)25-17-31(22-9-6-10-22)16-24(25)23-12-11-21(29)13-26(23)30/h3-5,7-8,11-13,18-19,22,24-25,34H,6,9-10,14-17H2,1-2H3/t18-,19+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440261
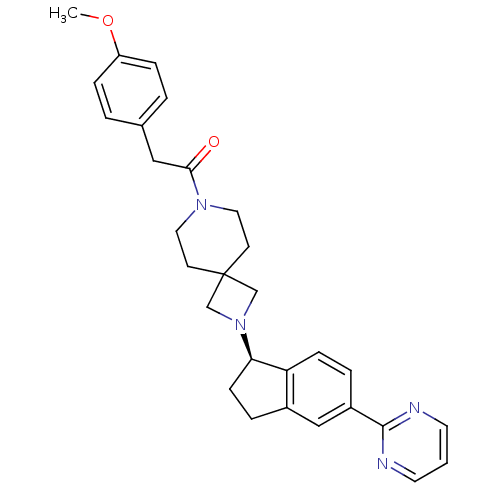
(CHEMBL2426677)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3ncccn3)CC2)cc1 |r| Show InChI InChI=1S/C29H32N4O2/c1-35-24-7-3-21(4-8-24)17-27(34)32-15-11-29(12-16-32)19-33(20-29)26-10-6-22-18-23(5-9-25(22)26)28-30-13-2-14-31-28/h2-5,7-9,13-14,18,26H,6,10-12,15-17,19-20H2,1H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061725
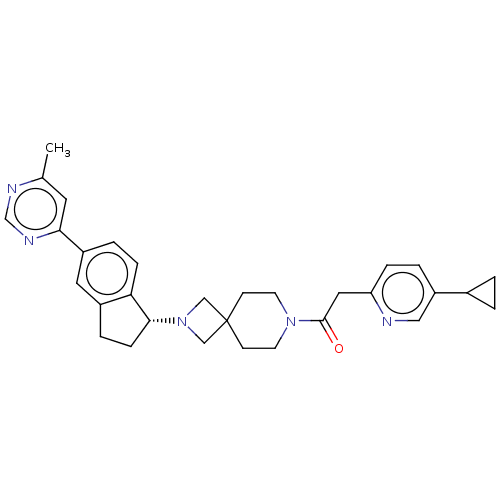
(CHEMBL3394195)Show SMILES Cc1cc(ncn1)-c1ccc2[C@@H](CCc2c1)N1CC2(C1)CCN(CC2)C(=O)Cc1ccc(cn1)C1CC1 |r| Show InChI InChI=1S/C31H35N5O/c1-21-14-28(34-20-33-21)24-5-8-27-23(15-24)6-9-29(27)36-18-31(19-36)10-12-35(13-11-31)30(37)16-26-7-4-25(17-32-26)22-2-3-22/h4-5,7-8,14-15,17,20,22,29H,2-3,6,9-13,16,18-19H2,1H3/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019926
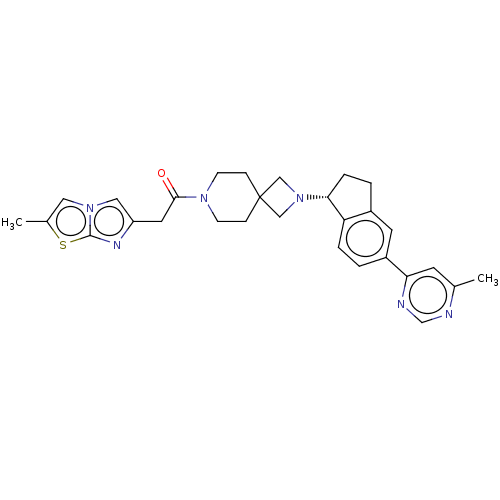
(CHEMBL3287218)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4cc(C)ncn4)CC3)nc2s1 |r| Show InChI InChI=1S/C29H32N6OS/c1-19-11-25(31-18-30-19)22-3-5-24-21(12-22)4-6-26(24)35-16-29(17-35)7-9-33(10-8-29)27(36)13-23-15-34-14-20(2)37-28(34)32-23/h3,5,11-12,14-15,18,26H,4,6-10,13,16-17H2,1-2H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019921

(CHEMBL3287213)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4ncccn4)CC3)nc2s1 |r| Show InChI InChI=1S/C28H30N6OS/c1-19-15-33-16-22(31-27(33)36-19)14-25(35)32-11-7-28(8-12-32)17-34(18-28)24-6-4-20-13-21(3-5-23(20)24)26-29-9-2-10-30-26/h2-3,5,9-10,13,15-16,24H,4,6-8,11-12,14,17-18H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human ghrelin receptor expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP-gamma-S b... |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019926

(CHEMBL3287218)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4cc(C)ncn4)CC3)nc2s1 |r| Show InChI InChI=1S/C29H32N6OS/c1-19-11-25(31-18-30-19)22-3-5-24-21(12-22)4-6-26(24)35-16-29(17-35)7-9-33(10-8-29)27(36)13-23-15-34-14-20(2)37-28(34)32-23/h3,5,11-12,14-15,18,26H,4,6-10,13,16-17H2,1-2H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human ghrelin receptor expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP-gamma-S b... |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315680
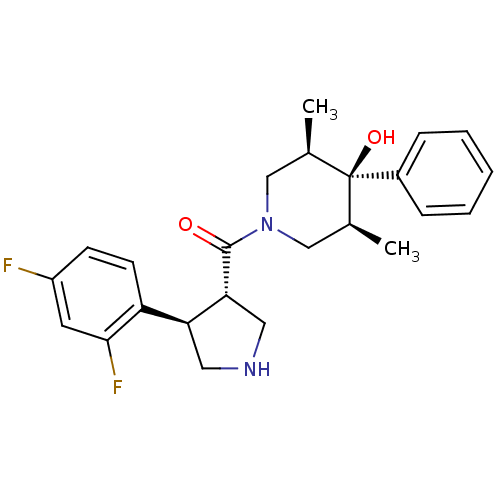
(((3S,4R)-4-(2,4-difluorophenyl)pyrrolidin-3-yl)((3...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CNC[C@H]1c1ccc(F)cc1F |r| Show InChI InChI=1S/C24H28F2N2O2/c1-15-13-28(14-16(2)24(15,30)17-6-4-3-5-7-17)23(29)21-12-27-11-20(21)19-9-8-18(25)10-22(19)26/h3-10,15-16,20-21,27,30H,11-14H2,1-2H3/t15-,16+,20-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440261

(CHEMBL2426677)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3ncccn3)CC2)cc1 |r| Show InChI InChI=1S/C29H32N4O2/c1-35-24-7-3-21(4-8-24)17-27(34)32-15-11-29(12-16-32)19-33(20-29)26-10-6-22-18-23(5-9-25(22)26)28-30-13-2-14-31-28/h2-5,7-9,13-14,18,26H,6,10-12,15-17,19-20H2,1H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378743
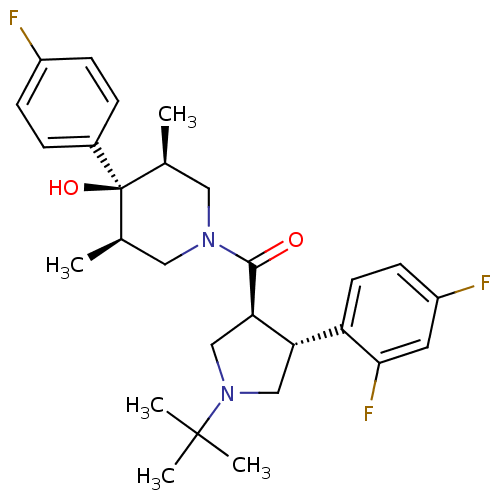
(CHEMBL1204061)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(F)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35F3N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315681
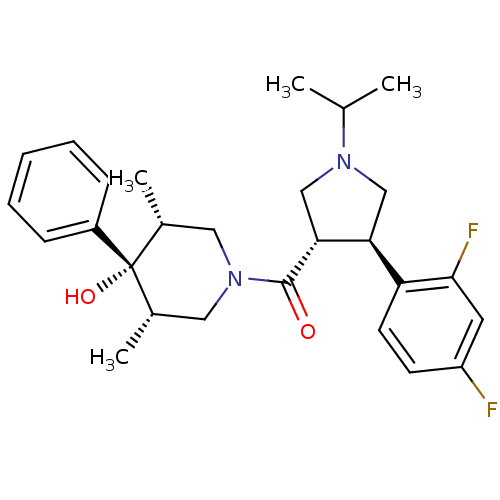
(((3S,4R)-4-(2,4-difluorophenyl)-1-isopropylpyrroli...)Show SMILES CC(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1C[C@H](C)[C@](O)([C@H](C)C1)c1ccccc1 |r| Show InChI InChI=1S/C27H34F2N2O2/c1-17(2)30-15-23(22-11-10-21(28)12-25(22)29)24(16-30)26(32)31-13-18(3)27(33,19(4)14-31)20-8-6-5-7-9-20/h5-12,17-19,23-24,33H,13-16H2,1-4H3/t18-,19+,23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440251
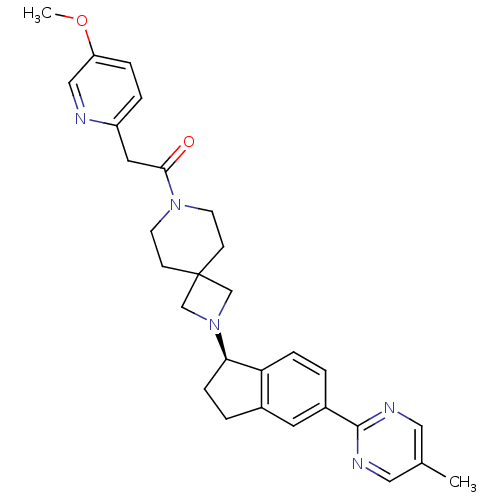
(CHEMBL2426687)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3ncc(C)cn3)CC2)nc1 |r| Show InChI InChI=1S/C29H33N5O2/c1-20-15-31-28(32-16-20)22-3-7-25-21(13-22)4-8-26(25)34-18-29(19-34)9-11-33(12-10-29)27(35)14-23-5-6-24(36-2)17-30-23/h3,5-7,13,15-17,26H,4,8-12,14,18-19H2,1-2H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315679

(((3R,4S)-1-cyclopropyl-4-(2,4-difluorophenyl)pyrro...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CC1 |r| Show InChI InChI=1S/C27H32F2N2O2/c1-17-13-31(14-18(2)27(17,33)19-6-4-3-5-7-19)26(32)24-16-30(21-9-10-21)15-23(24)22-11-8-20(28)12-25(22)29/h3-8,11-12,17-18,21,23-24,33H,9-10,13-16H2,1-2H3/t17-,18+,23-,24+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061717
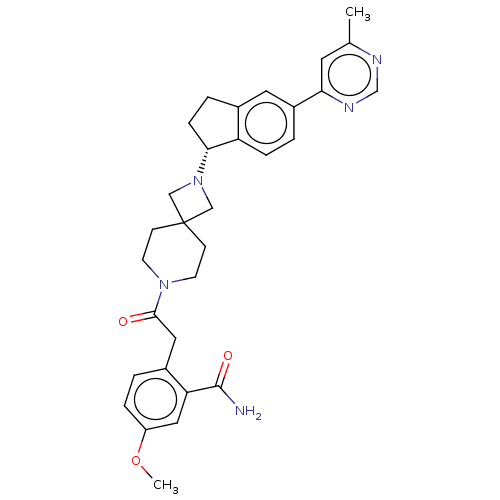
(CHEMBL3394201)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)c(c1)C(N)=O |r| Show InChI InChI=1S/C31H35N5O3/c1-20-13-27(34-19-33-20)23-4-7-25-21(14-23)5-8-28(25)36-17-31(18-36)9-11-35(12-10-31)29(37)15-22-3-6-24(39-2)16-26(22)30(32)38/h3-4,6-7,13-14,16,19,28H,5,8-12,15,17-18H2,1-2H3,(H2,32,38)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315673
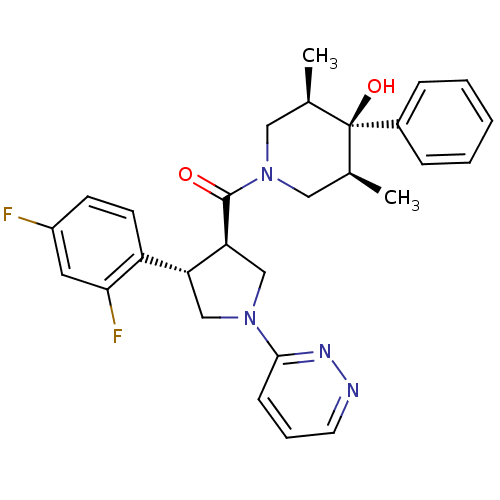
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridazin-3-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-14-34(15-19(2)28(18,36)20-7-4-3-5-8-20)27(35)24-17-33(26-9-6-12-31-32-26)16-23(24)22-11-10-21(29)13-25(22)30/h3-13,18-19,23-24,36H,14-17H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019922
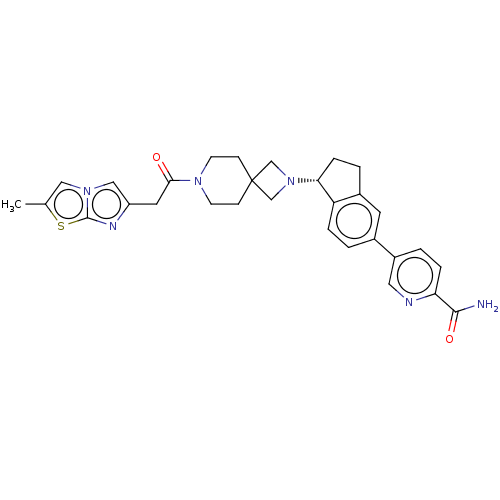
(CHEMBL3287214)Show SMILES Cc1cn2cc(CC(=O)N3CCC4(CN(C4)[C@@H]4CCc5cc(ccc45)-c4ccc(nc4)C(N)=O)CC3)nc2s1 |r| Show InChI InChI=1S/C30H32N6O2S/c1-19-15-35-16-23(33-29(35)39-19)13-27(37)34-10-8-30(9-11-34)17-36(18-30)26-7-4-21-12-20(2-5-24(21)26)22-3-6-25(28(31)38)32-14-22/h2-3,5-6,12,14-16,26H,4,7-11,13,17-18H2,1H3,(H2,31,38)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315673

(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridazin-3-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-14-34(15-19(2)28(18,36)20-7-4-3-5-8-20)27(35)24-17-33(26-9-6-12-31-32-26)16-23(24)22-11-10-21(29)13-25(22)30/h3-13,18-19,23-24,36H,14-17H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378748
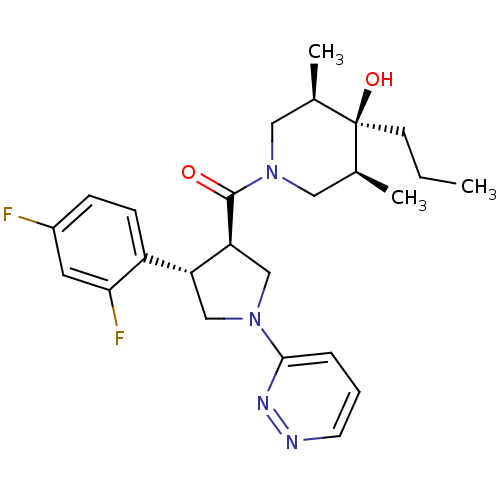
(CHEMBL1204054)Show SMILES CCC[C@]1(O)[C@@H](C)CN(C[C@H]1C)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C25H32F2N4O2/c1-4-9-25(33)16(2)12-31(13-17(25)3)24(32)21-15-30(23-6-5-10-28-29-23)14-20(21)19-8-7-18(26)11-22(19)27/h5-8,10-11,16-17,20-21,33H,4,9,12-15H2,1-3H3/t16-,17+,20-,21+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378747
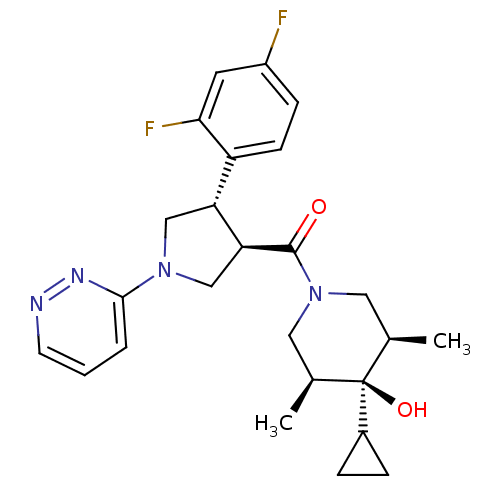
(CHEMBL1204056)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)C1CC1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C25H30F2N4O2/c1-15-11-31(12-16(2)25(15,33)17-5-6-17)24(32)21-14-30(23-4-3-9-28-29-23)13-20(21)19-8-7-18(26)10-22(19)27/h3-4,7-10,15-17,20-21,33H,5-6,11-14H2,1-2H3/t15-,16+,20-,21+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315692
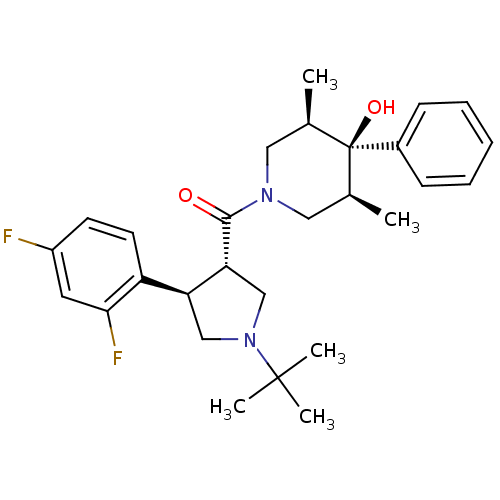
(CHEMBL1090488 | CHEMBL1204059)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H36F2N2O2/c1-18-14-31(15-19(2)28(18,34)20-9-7-6-8-10-20)26(33)24-17-32(27(3,4)5)16-23(24)22-12-11-21(29)13-25(22)30/h6-13,18-19,23-24,34H,14-17H2,1-5H3/t18-,19+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315691
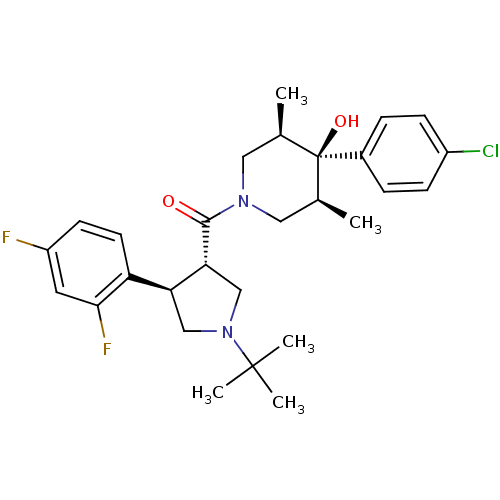
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(Cl)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35ClF2N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315669
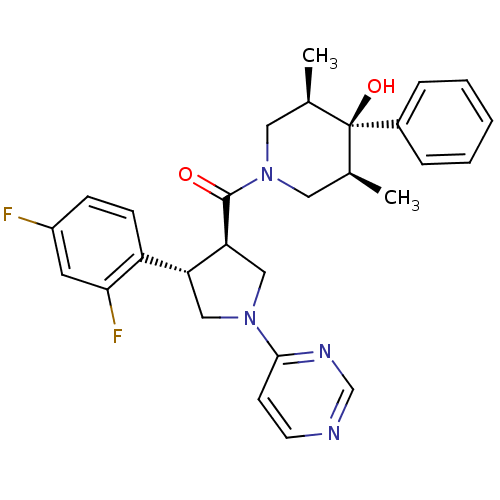
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyrimidin-4-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1ccncn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-13-34(14-19(2)28(18,36)20-6-4-3-5-7-20)27(35)24-16-33(26-10-11-31-17-32-26)15-23(24)22-9-8-21(29)12-25(22)30/h3-12,17-19,23-24,36H,13-16H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061720
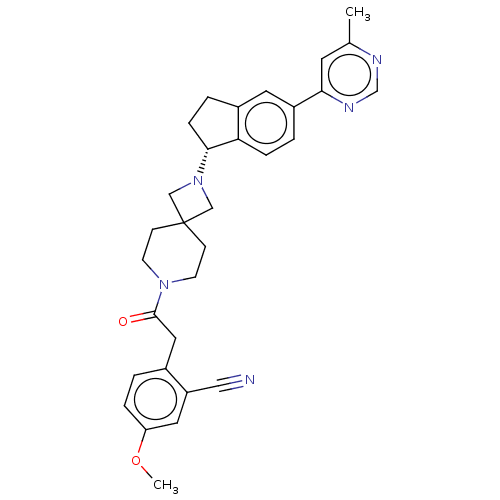
(CHEMBL3394199)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)c(c1)C#N |r| Show InChI InChI=1S/C31H33N5O2/c1-21-13-28(34-20-33-21)24-4-7-27-23(14-24)5-8-29(27)36-18-31(19-36)9-11-35(12-10-31)30(37)16-22-3-6-26(38-2)15-25(22)17-32/h3-4,6-7,13-15,20,29H,5,8-12,16,18-19H2,1-2H3/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019925
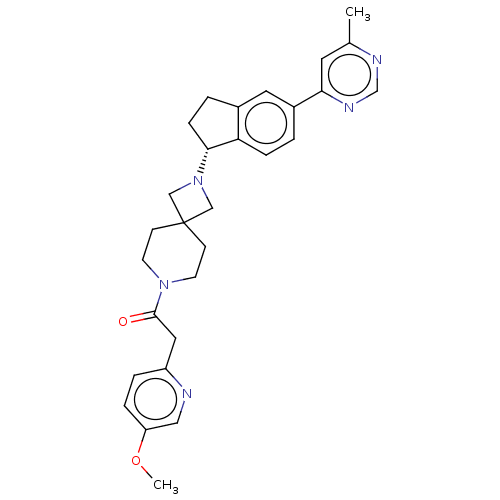
(CHEMBL3287217)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)nc1 |r| Show InChI InChI=1S/C29H33N5O2/c1-20-13-26(32-19-31-20)22-3-7-25-21(14-22)4-8-27(25)34-17-29(18-34)9-11-33(12-10-29)28(35)15-23-5-6-24(36-2)16-30-23/h3,5-7,13-14,16,19,27H,4,8-12,15,17-18H2,1-2H3/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019925

(CHEMBL3287217)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)nc1 |r| Show InChI InChI=1S/C29H33N5O2/c1-20-13-26(32-19-31-20)22-3-7-25-21(14-22)4-8-27(25)34-17-29(18-34)9-11-33(12-10-29)28(35)15-23-5-6-24(36-2)16-30-23/h3,5-7,13-14,16,19,27H,4,8-12,15,17-18H2,1-2H3/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019924
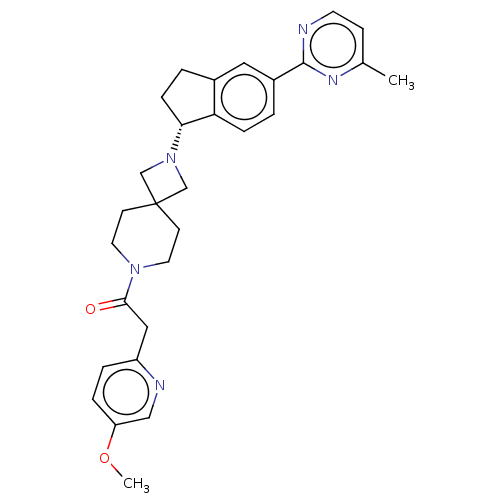
(CHEMBL3287216)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3nccc(C)n3)CC2)nc1 |r| Show InChI InChI=1S/C29H33N5O2/c1-20-9-12-30-28(32-20)22-3-7-25-21(15-22)4-8-26(25)34-18-29(19-34)10-13-33(14-11-29)27(35)16-23-5-6-24(36-2)17-31-23/h3,5-7,9,12,15,17,26H,4,8,10-11,13-14,16,18-19H2,1-2H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019925

(CHEMBL3287217)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)nc1 |r| Show InChI InChI=1S/C29H33N5O2/c1-20-13-26(32-19-31-20)22-3-7-25-21(14-22)4-8-27(25)34-17-29(18-34)9-11-33(12-10-29)28(35)15-23-5-6-24(36-2)16-30-23/h3,5-7,13-14,16,19,27H,4,8-12,15,17-18H2,1-2H3/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human ghrelin receptor expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP-gamma-S b... |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440261

(CHEMBL2426677)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3ncccn3)CC2)cc1 |r| Show InChI InChI=1S/C29H32N4O2/c1-35-24-7-3-21(4-8-24)17-27(34)32-15-11-29(12-16-32)19-33(20-29)26-10-6-22-18-23(5-9-25(22)26)28-30-13-2-14-31-28/h2-5,7-9,13-14,18,26H,6,10-12,15-17,19-20H2,1H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human ghrelin receptor expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP-gamma-S b... |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061713
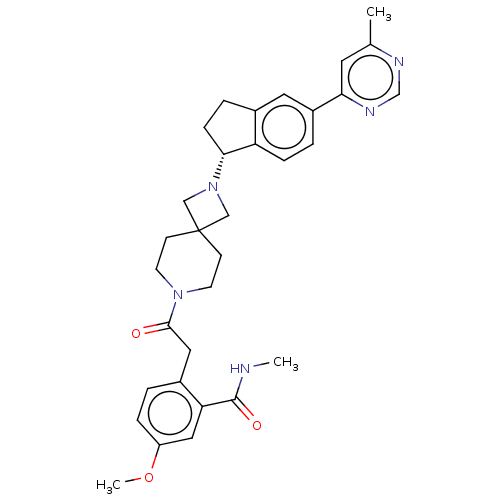
(CHEMBL3394205)Show SMILES CNC(=O)c1cc(OC)ccc1CC(=O)N1CCC2(CN(C2)[C@@H]2CCc3cc(ccc23)-c2cc(C)ncn2)CC1 |r| Show InChI InChI=1S/C32H37N5O3/c1-21-14-28(35-20-34-21)24-5-8-26-22(15-24)6-9-29(26)37-18-32(19-37)10-12-36(13-11-32)30(38)16-23-4-7-25(40-3)17-27(23)31(39)33-2/h4-5,7-8,14-15,17,20,29H,6,9-13,16,18-19H2,1-3H3,(H,33,39)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50029747
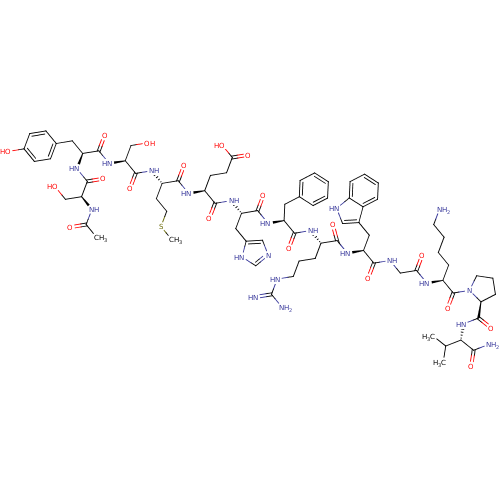
((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440259
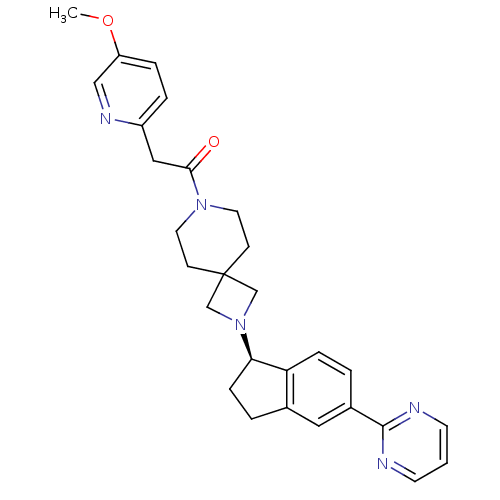
(CHEMBL2426680)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3ncccn3)CC2)nc1 |r| Show InChI InChI=1S/C28H31N5O2/c1-35-23-6-5-22(31-17-23)16-26(34)32-13-9-28(10-14-32)18-33(19-28)25-8-4-20-15-21(3-7-24(20)25)27-29-11-2-12-30-27/h2-3,5-7,11-12,15,17,25H,4,8-10,13-14,16,18-19H2,1H3/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human ghrelin receptor expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP-gamma-S b... |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315692

(CHEMBL1090488 | CHEMBL1204059)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H36F2N2O2/c1-18-14-31(15-19(2)28(18,34)20-9-7-6-8-10-20)26(33)24-17-32(27(3,4)5)16-23(24)22-12-11-21(29)13-25(22)30/h6-13,18-19,23-24,34H,14-17H2,1-5H3/t18-,19+,23-,24+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061712
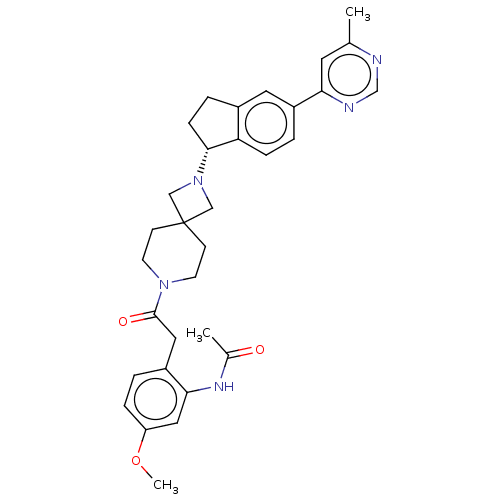
(CHEMBL3394206)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)c(NC(C)=O)c1 |r| Show InChI InChI=1S/C32H37N5O3/c1-21-14-28(34-20-33-21)24-5-8-27-23(15-24)6-9-30(27)37-18-32(19-37)10-12-36(13-11-32)31(39)16-25-4-7-26(40-3)17-29(25)35-22(2)38/h4-5,7-8,14-15,17,20,30H,6,9-13,16,18-19H2,1-3H3,(H,35,38)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50440259

(CHEMBL2426680)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3ncccn3)CC2)nc1 |r| Show InChI InChI=1S/C28H31N5O2/c1-35-23-6-5-22(31-17-23)16-26(34)32-13-9-28(10-14-32)18-33(19-28)25-8-4-20-15-21(3-7-24(20)25)27-29-11-2-12-30-27/h2-3,5-7,11-12,15,17,25H,4,8-10,13-14,16,18-19H2,1H3/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315676

(((3R,4S)-4-(2,4-difluorophenyl)-1-(tetrahydro-2H-p...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCOCC1 |r| Show InChI InChI=1S/C29H36F2N2O3/c1-19-15-33(16-20(2)29(19,35)21-6-4-3-5-7-21)28(34)26-18-32(23-10-12-36-13-11-23)17-25(26)24-9-8-22(30)14-27(24)31/h3-9,14,19-20,23,25-26,35H,10-13,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50378743

(CHEMBL1204061)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(F)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35F3N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315691

(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(Cl)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35ClF2N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061727
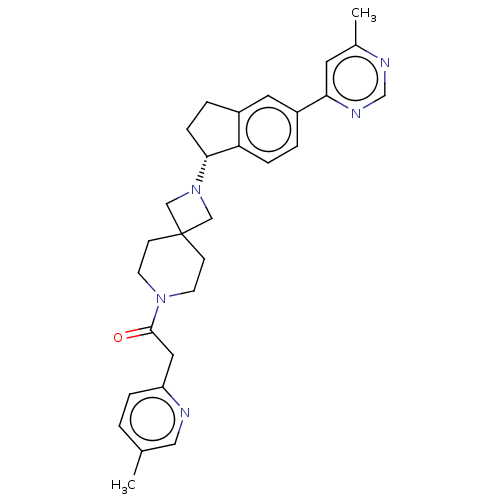
(CHEMBL3394193)Show SMILES Cc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)nc1 |r| Show InChI InChI=1S/C29H33N5O/c1-20-3-6-24(30-16-20)15-28(35)33-11-9-29(10-12-33)17-34(18-29)27-8-5-22-14-23(4-7-25(22)27)26-13-21(2)31-19-32-26/h3-4,6-7,13-14,16,19,27H,5,8-12,15,17-18H2,1-2H3/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50019924

(CHEMBL3287216)Show SMILES COc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3nccc(C)n3)CC2)nc1 |r| Show InChI InChI=1S/C29H33N5O2/c1-20-9-12-30-28(32-20)22-3-7-25-21(15-22)4-8-26(25)34-18-29(19-34)10-13-33(14-11-29)27(35)16-23-5-6-24(36-2)17-31-23/h3,5-7,9,12,15,17,26H,4,8,10-11,13-14,16,18-19H2,1-2H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human ghrelin receptor expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP-gamma-S b... |
ACS Med Chem Lett 5: 474-9 (2014)
Article DOI: 10.1021/ml400473x
BindingDB Entry DOI: 10.7270/Q2MS3VB3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315674

(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-3-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnc1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-7-4-3-5-8-21)28(35)26-18-33(23-9-6-12-32-14-23)17-25(26)24-11-10-22(30)13-27(24)31/h3-14,19-20,25-26,36H,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061721
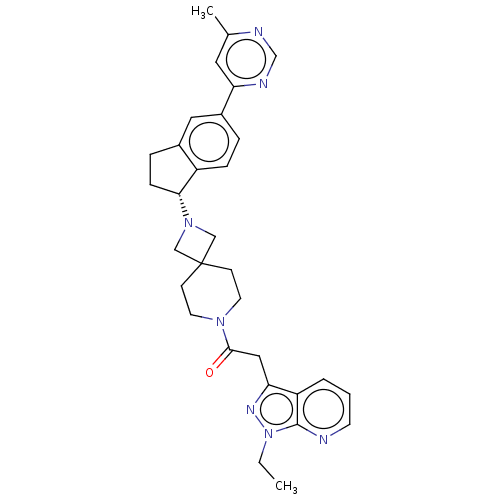
(CHEMBL3394198)Show SMILES CCn1nc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)c2cccnc12 |r| Show InChI InChI=1S/C31H35N7O/c1-3-38-30-25(5-4-12-32-30)27(35-38)17-29(39)36-13-10-31(11-14-36)18-37(19-31)28-9-7-22-16-23(6-8-24(22)28)26-15-21(2)33-20-34-26/h4-6,8,12,15-16,20,28H,3,7,9-11,13-14,17-19H2,1-2H3/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061724

(CHEMBL3394196)Show SMILES Cc1cc(ncn1)-c1ccc2[C@@H](CCc2c1)N1CC2(C1)CCN(CC2)C(=O)Cc1n[nH]c2ncccc12 |r| Show InChI InChI=1S/C29H31N7O/c1-19-13-24(32-18-31-19)21-4-6-22-20(14-21)5-7-26(22)36-16-29(17-36)8-11-35(12-9-29)27(37)15-25-23-3-2-10-30-28(23)34-33-25/h2-4,6,10,13-14,18,26H,5,7-9,11-12,15-17H2,1H3,(H,30,33,34)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50061726
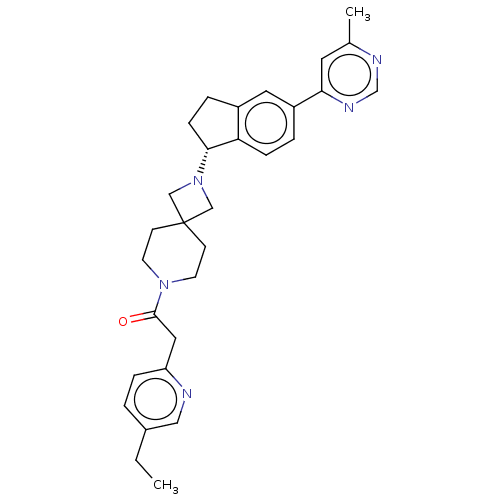
(CHEMBL3394194)Show SMILES CCc1ccc(CC(=O)N2CCC3(CN(C3)[C@@H]3CCc4cc(ccc34)-c3cc(C)ncn3)CC2)nc1 |r| Show InChI InChI=1S/C30H35N5O/c1-3-22-4-7-25(31-17-22)16-29(36)34-12-10-30(11-13-34)18-35(19-30)28-9-6-23-15-24(5-8-26(23)28)27-14-21(2)32-20-33-27/h4-5,7-8,14-15,17,20,28H,3,6,9-13,16,18-19H2,1-2H3/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method |
ACS Med Chem Lett 6: 156-61 (2015)
Article DOI: 10.1021/ml500414n
BindingDB Entry DOI: 10.7270/Q2N58P10 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315672
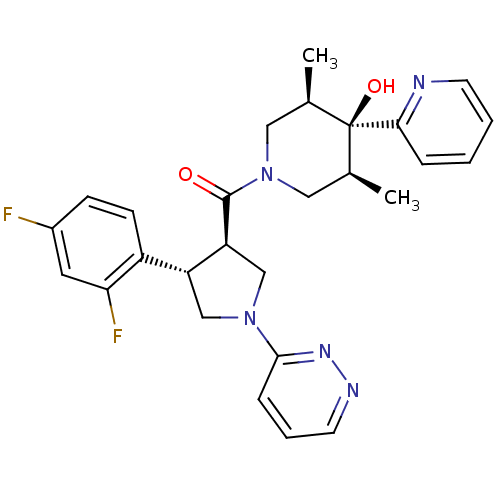
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridazin-3-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccn1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C27H29F2N5O2/c1-17-13-34(14-18(2)27(17,36)24-6-3-4-10-30-24)26(35)22-16-33(25-7-5-11-31-32-25)15-21(22)20-9-8-19(28)12-23(20)29/h3-12,17-18,21-22,36H,13-16H2,1-2H3/t17-,18+,21-,22+,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data