

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550593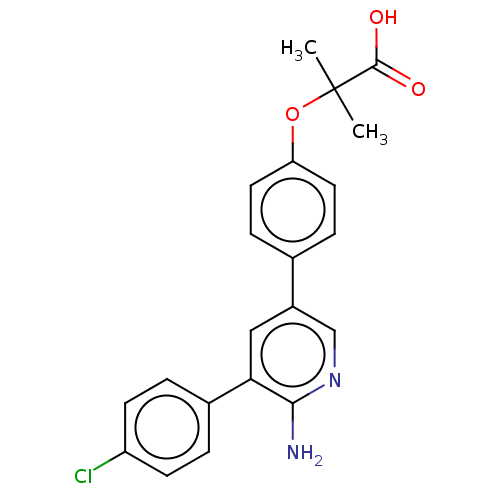 (CHEMBL4746566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550627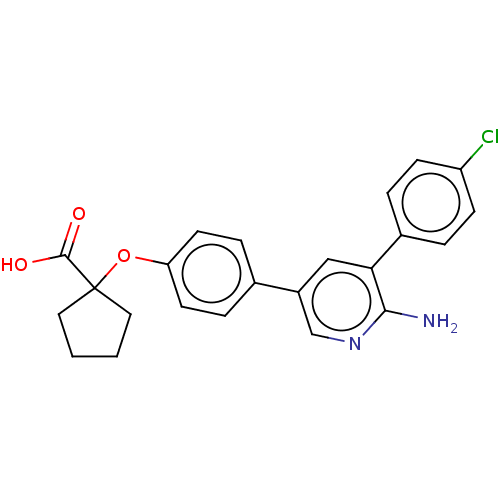 (CHEMBL4787152) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50550593 (CHEMBL4746566) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) in presence of 10 uM ATP | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18425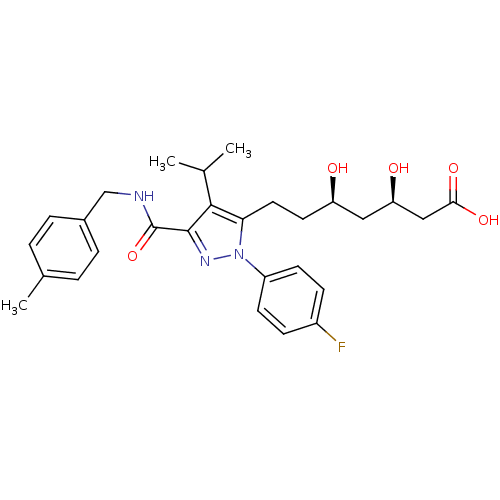 ((3R,5R)-7-[1-(4-fluorophenyl)-3-{[(4-methylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550624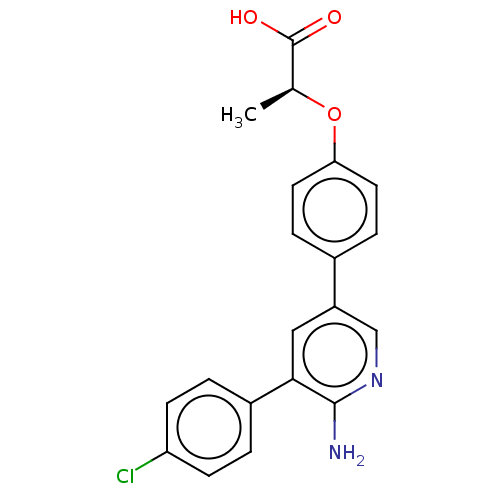 (CHEMBL4759743) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346286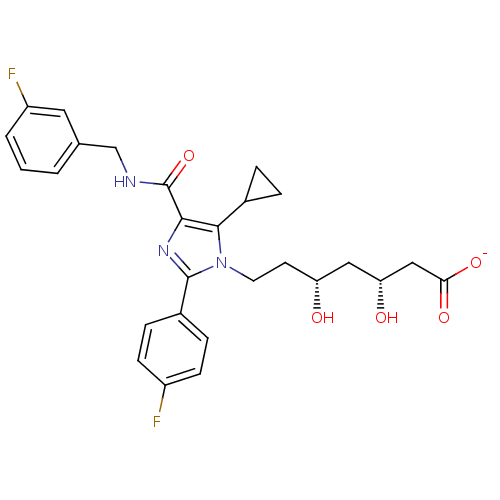 (CHEMBL1782559 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346288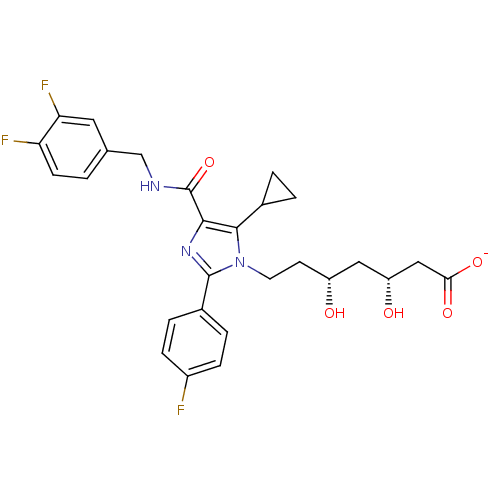 (CHEMBL1782560 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346289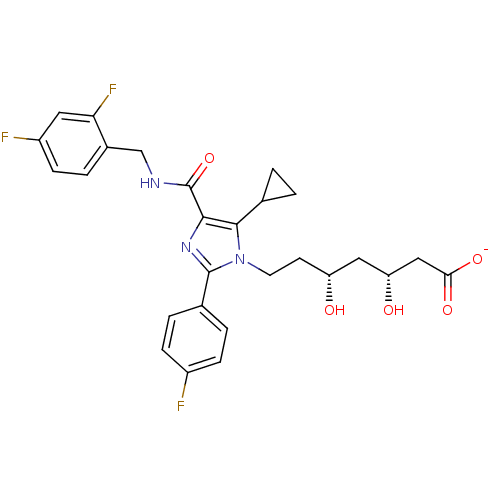 (CHEMBL1782561 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346283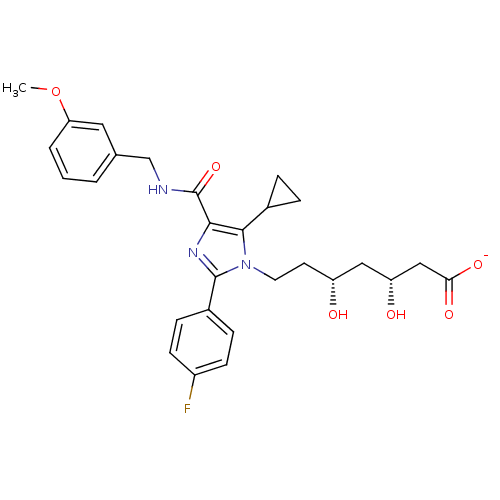 (CHEMBL1782556 | sodium(3R,5R)-7-(5-cyclopropyl-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346282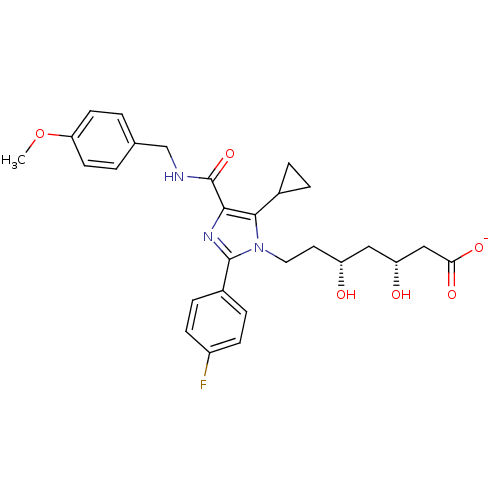 (CHEMBL1782555 | sodium(3R,5R)-7-(5-cyclopropyl-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Misshapen-like kinase 1 (Homo sapiens (Human)) | BDBM50550593 (CHEMBL4746566) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MINK (unknown origin) in presence of 10 uM ATP | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550629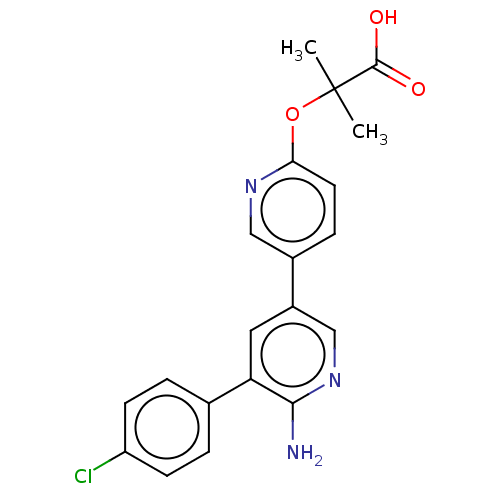 (CHEMBL4750540) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550595 (CHEMBL4783711) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346278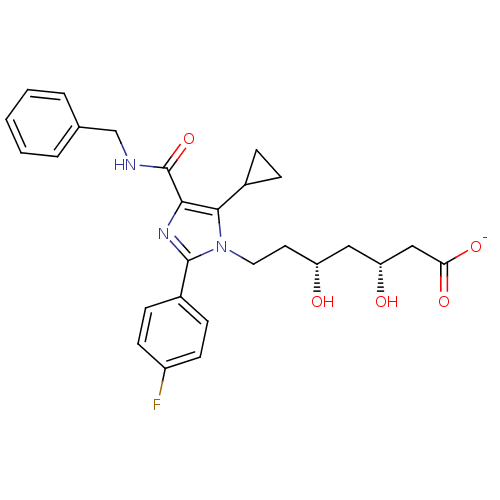 (CHEMBL1782551 | sodium(3R,5R)-7-(4-(benzylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550617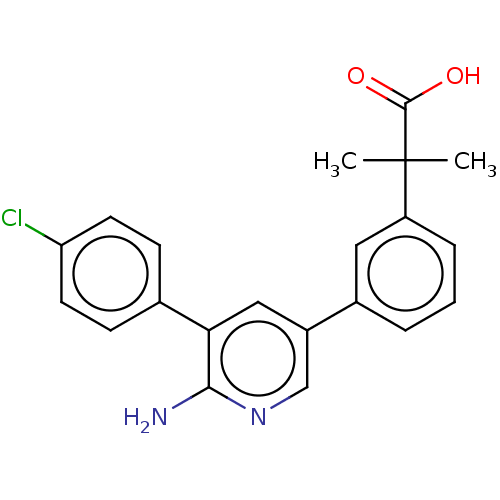 (CHEMBL4781045) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346287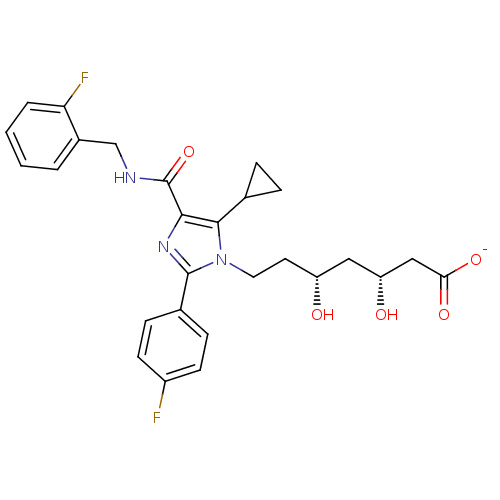 (CHEMBL1782062 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346279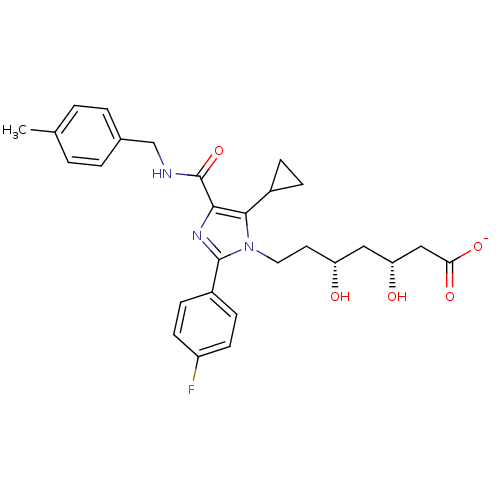 (CHEMBL1782552 | sodium(3R,5R)-7-(5-cyclopropyl-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550619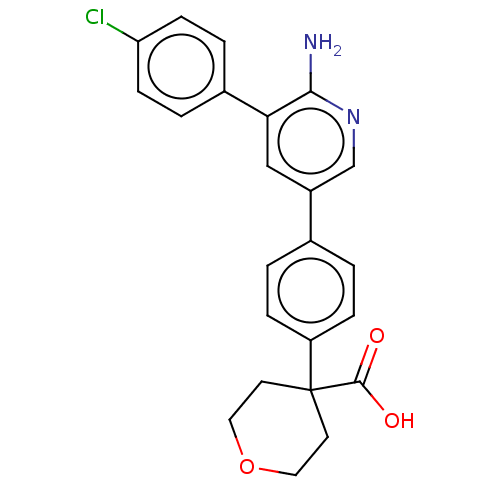 (CHEMBL4787142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550623 (CHEMBL4779507) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550628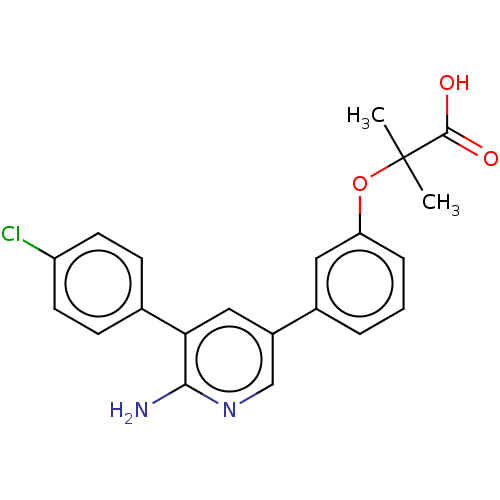 (CHEMBL4740167) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550600 (CHEMBL4745446) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550597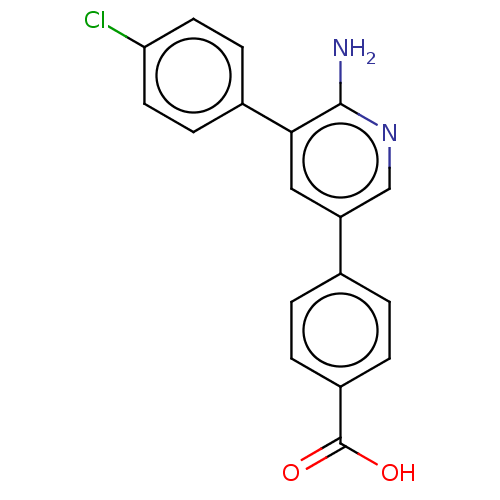 (CHEMBL4757082) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550598 (CHEMBL4751017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550594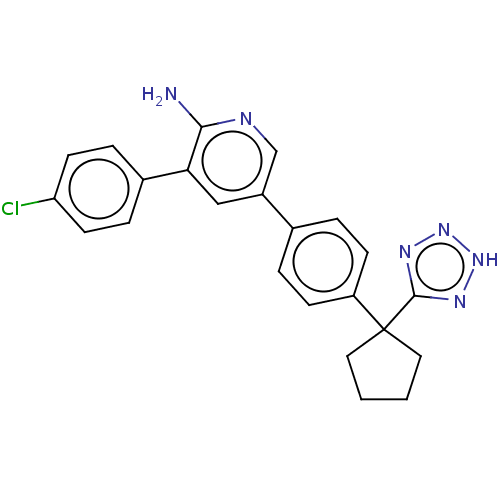 (CHEMBL4777705) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31149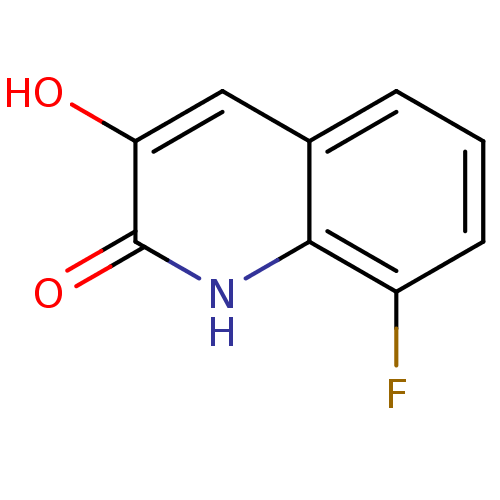 (3-hydroxyquinolin-2(1H)-one, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31173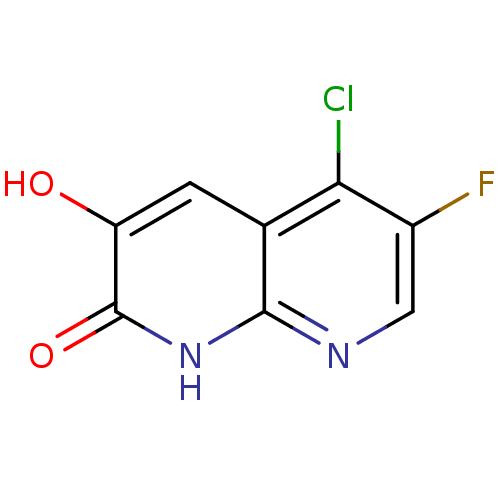 (naphthyridinone analog., 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18372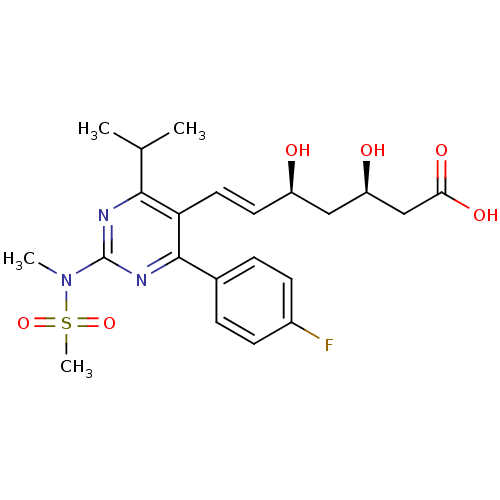 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346285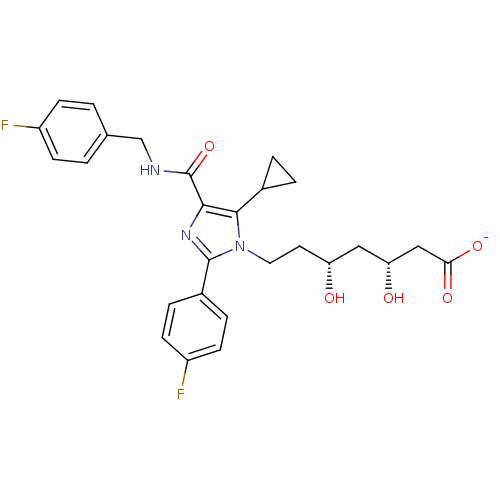 (CHEMBL1782558 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550607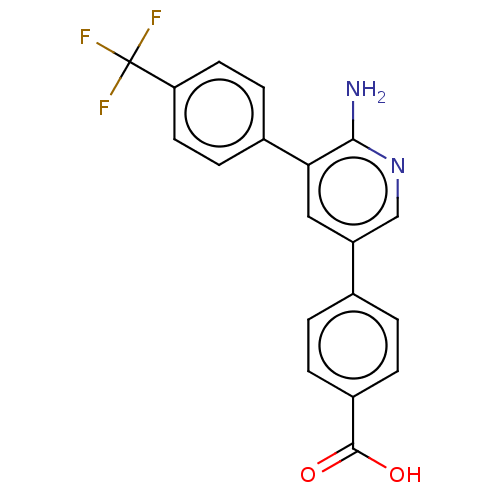 (CHEMBL4788172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550610 (CHEMBL4800212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550616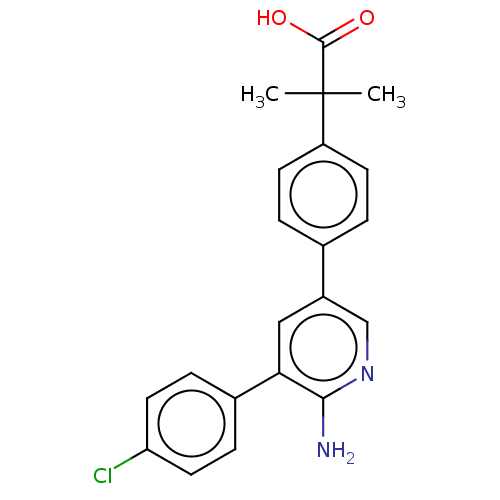 (CHEMBL4760299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346291 (CHEMBL1782563 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50134771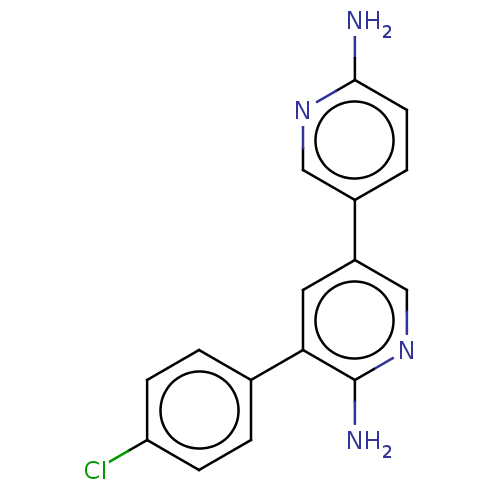 (CHEMBL3754515) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50134771 (CHEMBL3754515) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAP4K4 catalytic domain in presence of 10 uM ATP (Km) by FRET assay | ACS Med Chem Lett 6: 1128-33 (2015) Article DOI: 10.1021/acsmedchemlett.5b00215 BindingDB Entry DOI: 10.7270/Q2028TC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31148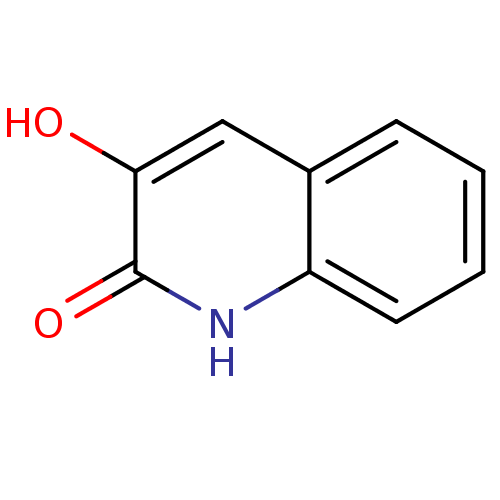 (3-hydroxyquinolin-2(1H)-one, 2 | US9701638, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Rattus norvegicus (rat)) | BDBM31173 (naphthyridinone analog., 27) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31156 (3-hydroxyquinolin-2(1H)-one, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550602 (CHEMBL4790042) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346290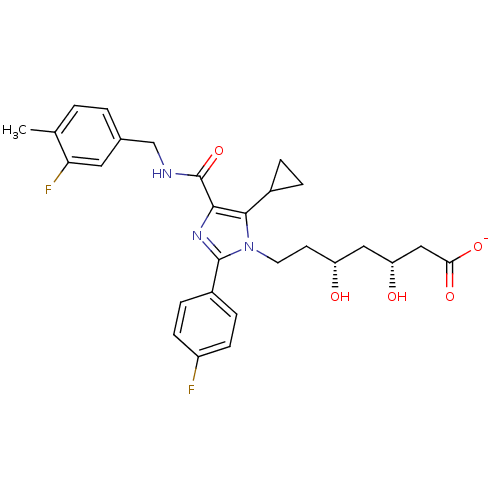 (CHEMBL1782562 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50138160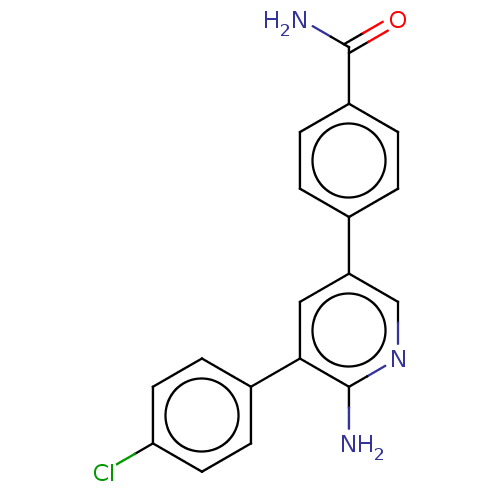 (CHEMBL3753424) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550618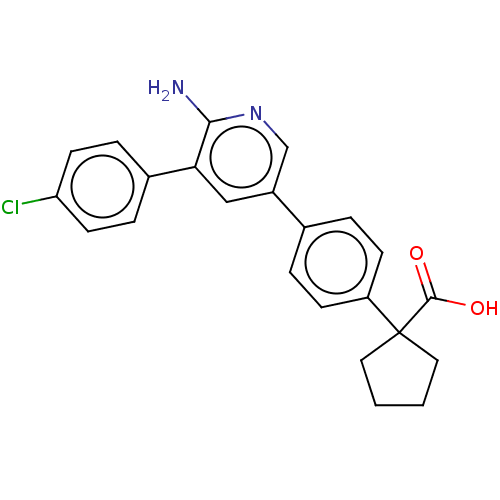 (CHEMBL4778859) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346293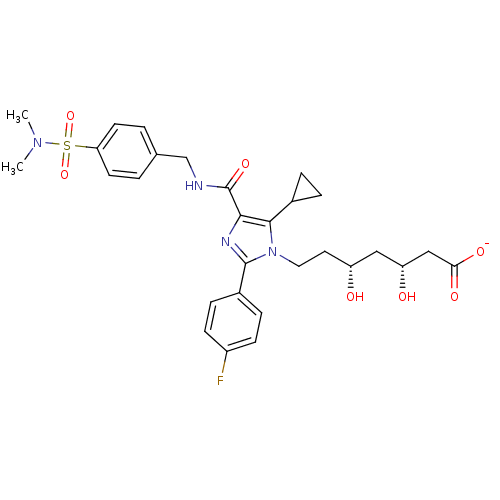 (CHEMBL1782565 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346281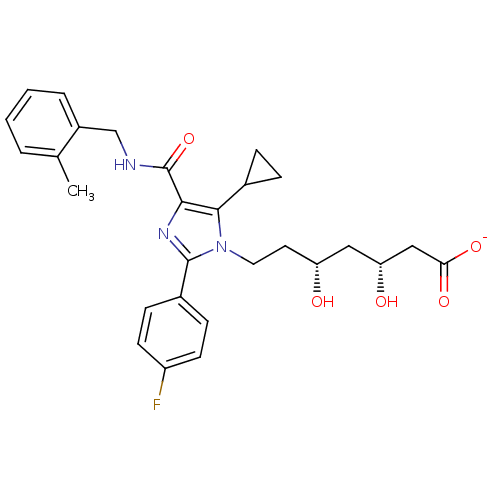 (CHEMBL1782554 | sodium(3R,5R)-7-(5-cyclopropyl-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31172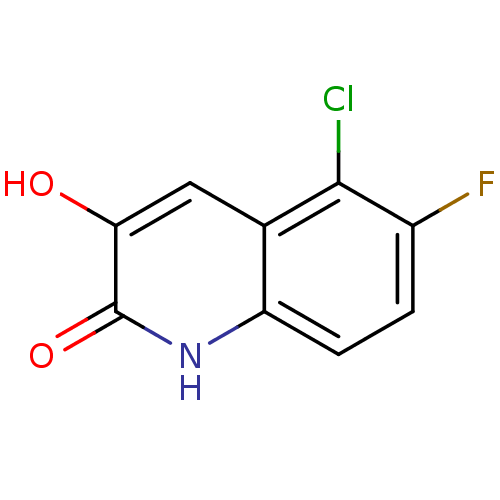 (3-hydroxyquinolin-2(1H)-one, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550601 (CHEMBL4780690) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 4 (Homo sapiens (Human)) | BDBM50550593 (CHEMBL4746566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged MAP4K4 catalytic domain (1 to 328 residues) expressed in baculovirus expression system pre-incubated for 3... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 385 total ) | Next | Last >> |