| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sphingosine 1-phosphate receptor 2 |
|---|
| Ligand | BDBM50149566 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1558242 (CHEMBL3772376) |
|---|
| EC50 | <31623±n/a nM |
|---|
| Citation |  Demont, EH; Bailey, JM; Bit, RA; Brown, JA; Campbell, CA; Deeks, N; Dowell, SJ; Eldred, C; Gaskin, P; Gray, JR; Haynes, A; Hirst, DJ; Holmes, DS; Kumar, U; Morse, MA; Osborne, GJ; Renaux, JF; Seal, GA; Smethurst, CA; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of Tetrahydropyrazolopyridine as Sphingosine 1-Phosphate Receptor 3 (S1P3)-Sparing S1P1 Agonists Active at Low Oral Doses. J Med Chem59:1003-20 (2016) [PubMed] Article Demont, EH; Bailey, JM; Bit, RA; Brown, JA; Campbell, CA; Deeks, N; Dowell, SJ; Eldred, C; Gaskin, P; Gray, JR; Haynes, A; Hirst, DJ; Holmes, DS; Kumar, U; Morse, MA; Osborne, GJ; Renaux, JF; Seal, GA; Smethurst, CA; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of Tetrahydropyrazolopyridine as Sphingosine 1-Phosphate Receptor 3 (S1P3)-Sparing S1P1 Agonists Active at Low Oral Doses. J Med Chem59:1003-20 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sphingosine 1-phosphate receptor 2 |
|---|
| Name: | Sphingosine 1-phosphate receptor 2 |
|---|
| Synonyms: | EDG5 | S1P2 | S1PR2 | S1PR2_HUMAN | Sphingosine 1-phosphate receptor | Sphingosine 1-phosphate receptor Edg-5 | Sphingosine-1-phosphate receptor 2 | ndothelial differentiation G-protein coupled receptor 5 |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 38883.16 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Membranes isolated from S1P2-transfected CHO cells were used in ligand binding assay. |
|---|
| Residue: | 353 |
|---|
| Sequence: | MGSLYSEYLNPNKVQEHYNYTKETLETQETTSRQVASAFIVILCCAIVVENLLVLIAVAR
NSKFHSAMYLFLGNLAASDLLAGVAFVANTLLSGSVTLRLTPVQWFAREGSAFITLSASV
FSLLAIAIERHVAIAKVKLYGSDKSCRMLLLIGASWLISLVLGGLPILGWNCLGHLEACS
TVLPLYAKHYVLCVVTIFSIILLAIVALYVRIYCVVRSSHADMAAPQTLALLKTVTIVLG
VFIVCWLPAFSILLLDYACPVHSCPILYKAHYFFAVSTLNSLLNPVIYTWRSRDLRREVL
RPLQCWRPGVGVQGRRRGGTPGHHLLPLRSSSSLERGMHMPTSPTFLEGNTVV
|
|
|
|---|
| BDBM50149566 |
|---|
| n/a |
|---|
| Name | BDBM50149566 |
|---|
| Synonyms: | CHEMBL3769933 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H24ClN5O3S |
|---|
| Mol. Mass. | 461.965 |
|---|
| SMILES | CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C |
|---|
| Structure | 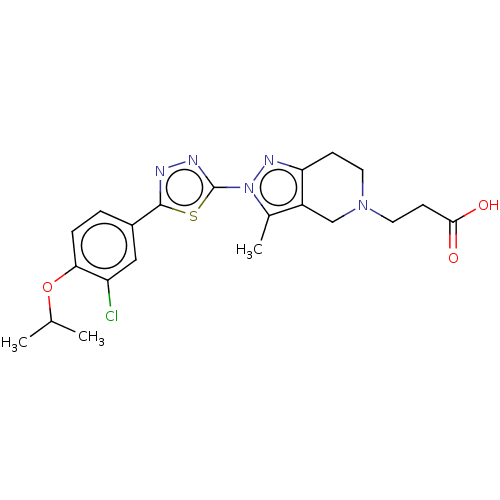
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Demont, EH; Bailey, JM; Bit, RA; Brown, JA; Campbell, CA; Deeks, N; Dowell, SJ; Eldred, C; Gaskin, P; Gray, JR; Haynes, A; Hirst, DJ; Holmes, DS; Kumar, U; Morse, MA; Osborne, GJ; Renaux, JF; Seal, GA; Smethurst, CA; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of Tetrahydropyrazolopyridine as Sphingosine 1-Phosphate Receptor 3 (S1P3)-Sparing S1P1 Agonists Active at Low Oral Doses. J Med Chem59:1003-20 (2016) [PubMed] Article
Demont, EH; Bailey, JM; Bit, RA; Brown, JA; Campbell, CA; Deeks, N; Dowell, SJ; Eldred, C; Gaskin, P; Gray, JR; Haynes, A; Hirst, DJ; Holmes, DS; Kumar, U; Morse, MA; Osborne, GJ; Renaux, JF; Seal, GA; Smethurst, CA; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of Tetrahydropyrazolopyridine as Sphingosine 1-Phosphate Receptor 3 (S1P3)-Sparing S1P1 Agonists Active at Low Oral Doses. J Med Chem59:1003-20 (2016) [PubMed] Article