 Found 129 hits with Last Name = 'osborne' and Initial = 'gj'
Found 129 hits with Last Name = 'osborne' and Initial = 'gj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419206
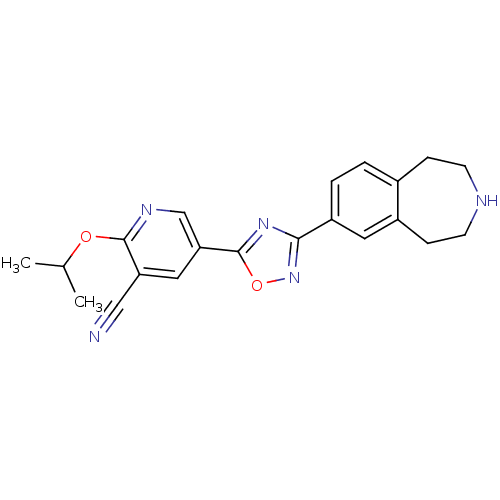
(CHEMBL1836170)Show SMILES CC(C)Oc1ncc(cc1C#N)-c1nc(no1)-c1ccc2CCNCCc2c1 Show InChI InChI=1S/C21H21N5O2/c1-13(2)27-20-17(11-22)10-18(12-24-20)21-25-19(26-28-21)16-4-3-14-5-7-23-8-6-15(14)9-16/h3-4,9-10,12-13,23H,5-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50354138
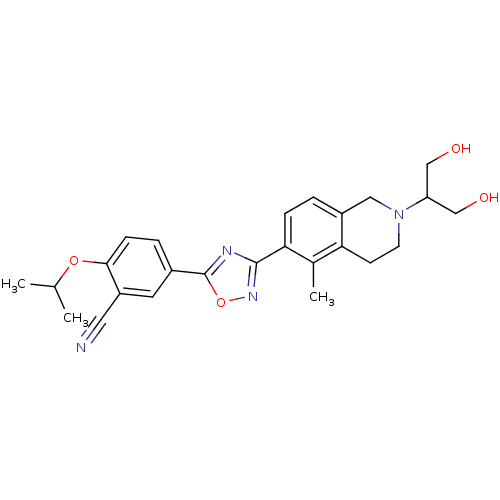
(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419207
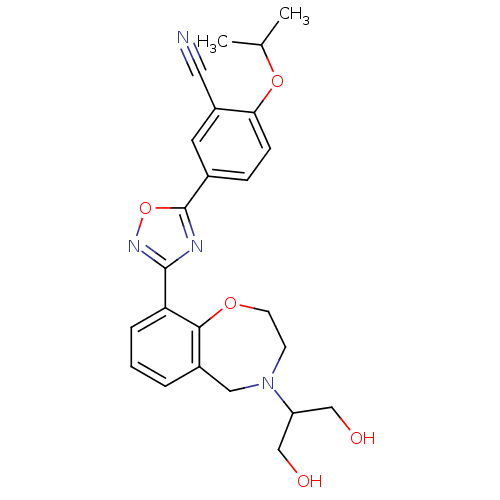
(CHEMBL1836212)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1cccc2CN(CCOc12)C(CO)CO Show InChI InChI=1S/C24H26N4O5/c1-15(2)32-21-7-6-16(10-18(21)11-25)24-26-23(27-33-24)20-5-3-4-17-12-28(19(13-29)14-30)8-9-31-22(17)20/h3-7,10,15,19,29-30H,8-9,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using vivid green as substrate |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419201

(CHEMBL1836171)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CC(=O)N[C@@H](C)CO)CCc2c1C |r| Show InChI InChI=1S/C27H31N5O4/c1-16(2)35-24-8-6-19(11-21(24)12-28)27-30-26(31-36-27)23-7-5-20-13-32(10-9-22(20)18(23)4)14-25(34)29-17(3)15-33/h5-8,11,16-17,33H,9-10,13-15H2,1-4H3,(H,29,34)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419204

(CHEMBL1836213)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1cnc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C24H27N5O4/c1-14(2)32-22-5-4-16(8-17(22)9-25)24-27-23(28-33-24)20-10-26-21-11-29(18(12-30)13-31)7-6-19(21)15(20)3/h4-5,8,10,14,18,30-31H,6-7,11-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using vivid red as substrate |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419205
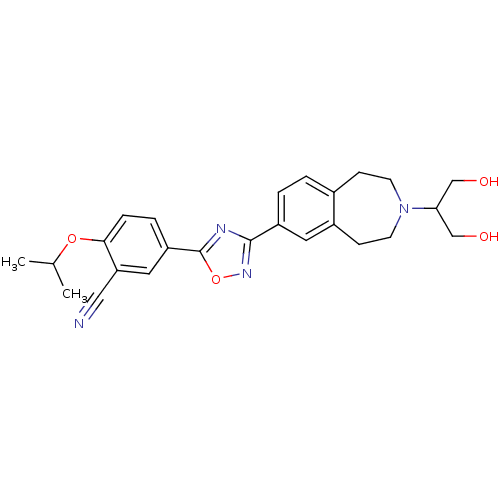
(CHEMBL1836172)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CCN(CCc2c1)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-16(2)32-23-6-5-20(12-21(23)13-26)25-27-24(28-33-25)19-4-3-17-7-9-29(22(14-30)15-31)10-8-18(17)11-19/h3-6,11-12,16,22,30-31H,7-10,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419203
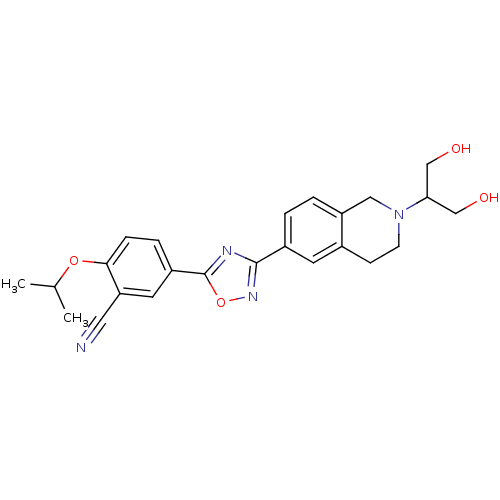
(CHEMBL1836214)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1)C(CO)CO Show InChI InChI=1S/C24H26N4O4/c1-15(2)31-22-6-5-18(10-20(22)11-25)24-26-23(27-32-24)17-3-4-19-12-28(21(13-29)14-30)8-7-16(19)9-17/h3-6,9-10,15,21,29-30H,7-8,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50149566
(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) coexpressed in Escherichia coli with human NADPH reductase using 3-butyryl-7-methoxycoumarin as substrate afte... |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) coexpressed in Escherichia coli with human NADPH reductase using 7-methoxy-4-trifluoromethylcoumarin-3-acetic a... |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by VG metabolism assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by VR metabolism assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) coexpressed in Escherichia coli with human NADPH reductase using 4-methylaminomethyl-7-methoyxycoumarin as subs... |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) coexpressed in Escherichia coli with human NADPH reductase using ethoxyresorufin as substrate after 10 mins by ... |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149581
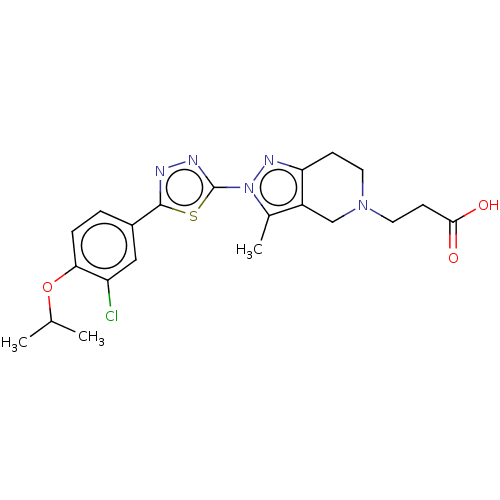
(CHEMBL3771149)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)N1CCOc2c(CCC(O)=O)cccc12 Show InChI InChI=1S/C22H22ClN3O4S/c1-13(2)30-18-8-6-15(12-16(18)23)21-24-25-22(31-21)26-10-11-29-20-14(7-9-19(27)28)4-3-5-17(20)26/h3-6,8,12-13H,7,9-11H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149582

(CHEMBL3770821)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)N1CCc2cc(CCC(O)=O)ccc12 Show InChI InChI=1S/C22H22ClN3O3S/c1-13(2)29-19-7-5-16(12-17(19)23)21-24-25-22(30-21)26-10-9-15-11-14(3-6-18(15)26)4-8-20(27)28/h3,5-7,11-13H,4,8-10H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149572
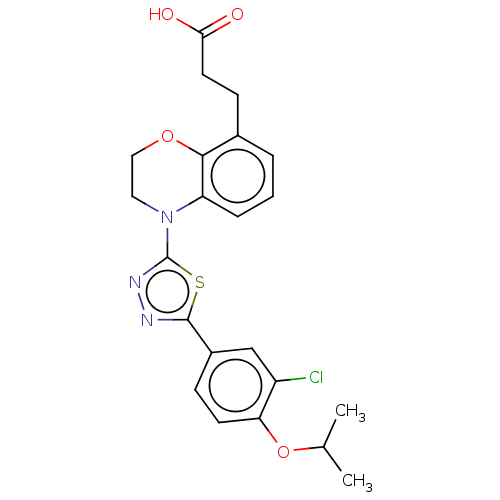
(CHEMBL3771092)Show InChI InChI=1S/C17H15ClN2OS/c1-11(2)21-15-9-8-13(10-14(15)18)17-20-19-16(22-17)12-6-4-3-5-7-12/h3-11H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149573
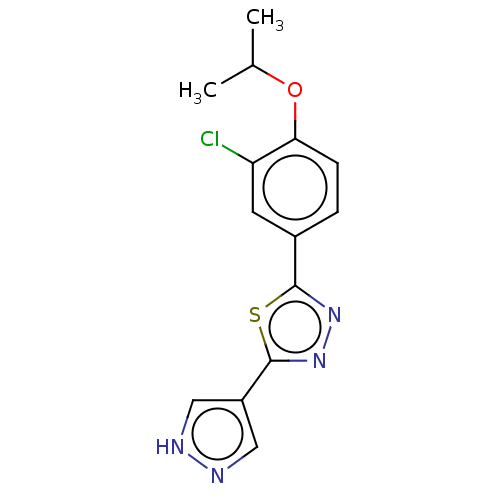
(CHEMBL3769742)Show InChI InChI=1S/C14H13ClN4OS/c1-8(2)20-12-4-3-9(5-11(12)15)13-18-19-14(21-13)10-6-16-17-7-10/h3-8H,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149583
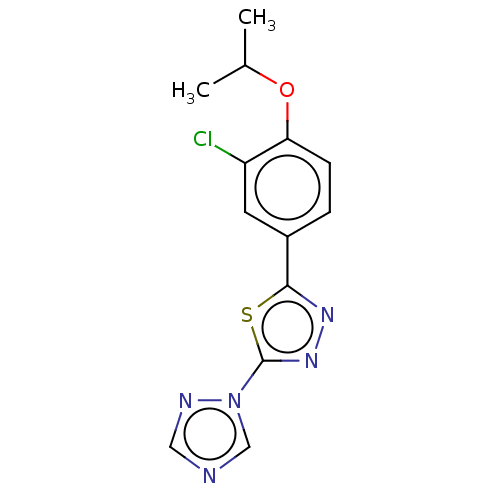
(CHEMBL3770492)Show InChI InChI=1S/C13H12ClN5OS/c1-8(2)20-11-4-3-9(5-10(11)14)12-17-18-13(21-12)19-7-15-6-16-19/h3-8H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149584
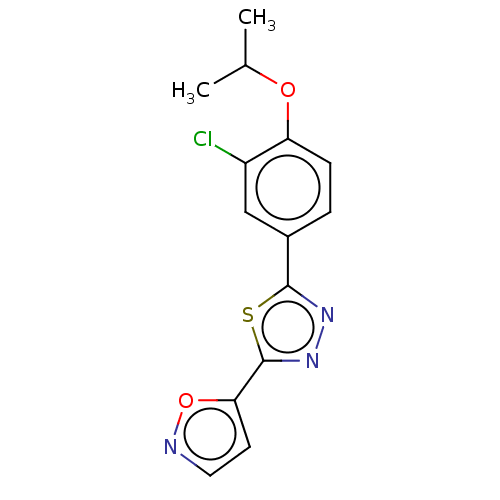
(CHEMBL3770423)Show InChI InChI=1S/C14H12ClN3O2S/c1-8(2)19-11-4-3-9(7-10(11)15)13-17-18-14(21-13)12-5-6-16-20-12/h3-8H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149585
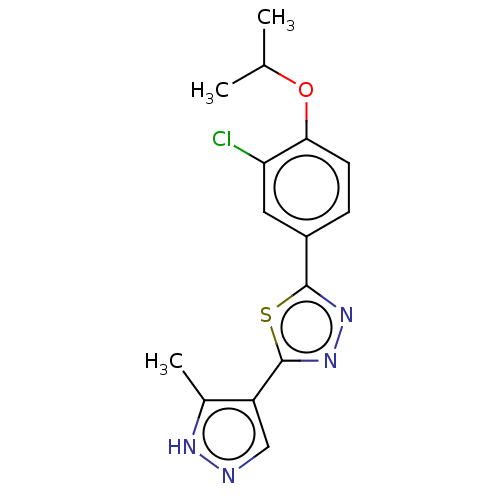
(CHEMBL3770352)Show InChI InChI=1S/C15H15ClN4OS/c1-8(2)21-13-5-4-10(6-12(13)16)14-19-20-15(22-14)11-7-17-18-9(11)3/h4-8H,1-3H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149586
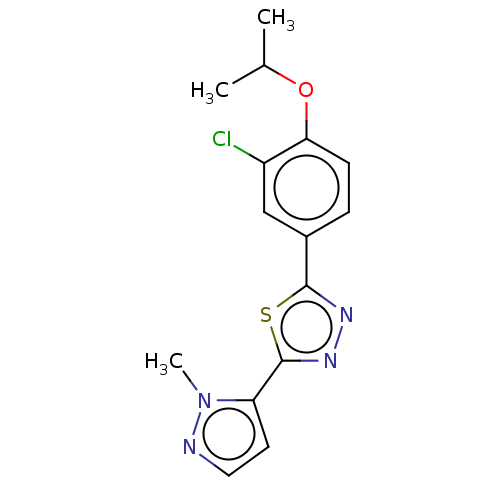
(CHEMBL3770390)Show InChI InChI=1S/C15H15ClN4OS/c1-9(2)21-13-5-4-10(8-11(13)16)14-18-19-15(22-14)12-6-7-17-20(12)3/h4-9H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149587

(CHEMBL3770587)Show InChI InChI=1S/C16H16ClN3OS2/c1-8(2)21-13-6-5-11(7-12(13)17)15-19-20-16(23-15)14-9(3)18-10(4)22-14/h5-8H,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149588
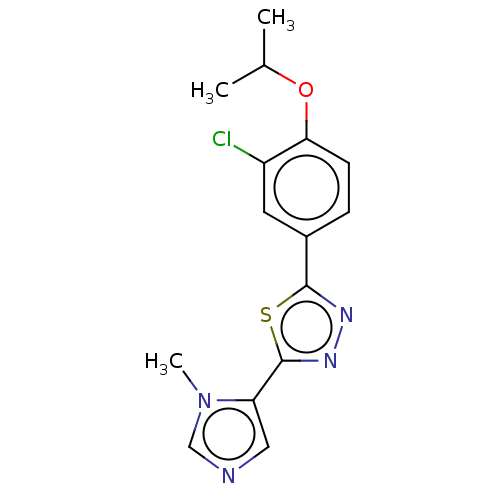
(CHEMBL3770302)Show InChI InChI=1S/C15H15ClN4OS/c1-9(2)21-13-5-4-10(6-11(13)16)14-18-19-15(22-14)12-7-17-8-20(12)3/h4-9H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149589

(CHEMBL3770772)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-c1cnn(CC(O)=O)c1 Show InChI InChI=1S/C16H15ClN4O3S/c1-9(2)24-13-4-3-10(5-12(13)17)15-19-20-16(25-15)11-6-18-21(7-11)8-14(22)23/h3-7,9H,8H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149555
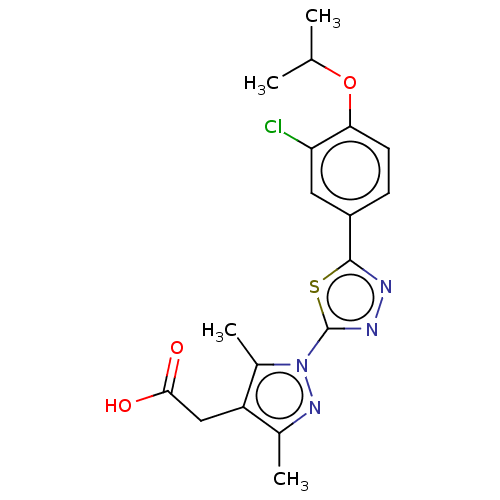
(CHEMBL3770278)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc(C)c(CC(O)=O)c1C Show InChI InChI=1S/C18H19ClN4O3S/c1-9(2)26-15-6-5-12(7-14(15)19)17-20-21-18(27-17)23-11(4)13(8-16(24)25)10(3)22-23/h5-7,9H,8H2,1-4H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149556
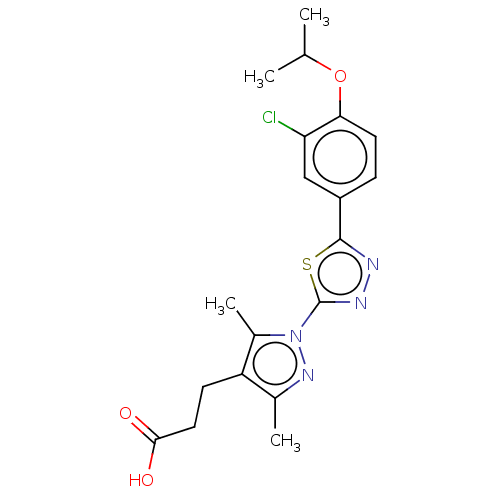
(CHEMBL3770208)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc(C)c(CCC(O)=O)c1C Show InChI InChI=1S/C19H21ClN4O3S/c1-10(2)27-16-7-5-13(9-15(16)20)18-21-22-19(28-18)24-12(4)14(11(3)23-24)6-8-17(25)26/h5,7,9-10H,6,8H2,1-4H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149590
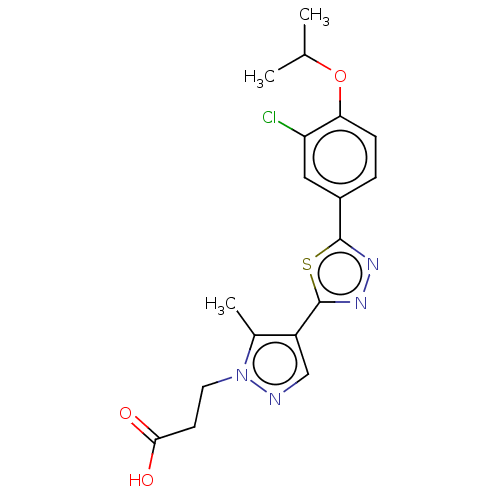
(CHEMBL3769703)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-c1cnn(CCC(O)=O)c1C Show InChI InChI=1S/C18H19ClN4O3S/c1-10(2)26-15-5-4-12(8-14(15)19)17-21-22-18(27-17)13-9-20-23(11(13)3)7-6-16(24)25/h4-5,8-10H,6-7H2,1-3H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149591
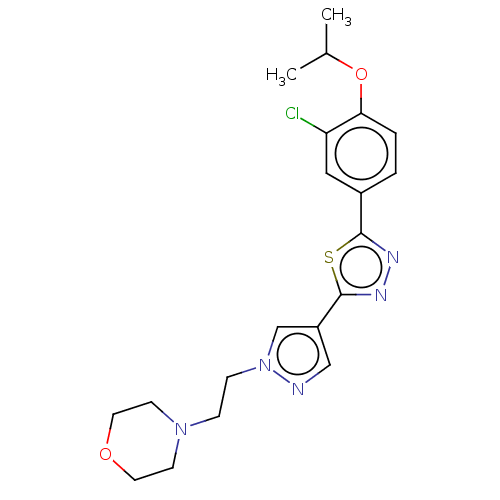
(CHEMBL3769524)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-c1cnn(CCN2CCOCC2)c1 Show InChI InChI=1S/C20H24ClN5O2S/c1-14(2)28-18-4-3-15(11-17(18)21)19-23-24-20(29-19)16-12-22-26(13-16)6-5-25-7-9-27-10-8-25/h3-4,11-14H,5-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149583

(CHEMBL3770492)Show InChI InChI=1S/C13H12ClN5OS/c1-8(2)20-11-4-3-9(5-10(11)14)12-17-18-13(21-12)19-7-15-6-16-19/h3-8H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149584

(CHEMBL3770423)Show InChI InChI=1S/C14H12ClN3O2S/c1-8(2)19-11-4-3-9(7-10(11)15)13-17-18-14(21-13)12-5-6-16-20-12/h3-8H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149585

(CHEMBL3770352)Show InChI InChI=1S/C15H15ClN4OS/c1-8(2)21-13-5-4-10(6-12(13)16)14-19-20-15(22-14)11-7-17-18-9(11)3/h4-8H,1-3H3,(H,17,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149586

(CHEMBL3770390)Show InChI InChI=1S/C15H15ClN4OS/c1-9(2)21-13-5-4-10(8-11(13)16)14-18-19-15(22-14)12-6-7-17-20(12)3/h4-9H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149587

(CHEMBL3770587)Show InChI InChI=1S/C16H16ClN3OS2/c1-8(2)21-13-6-5-11(7-12(13)17)15-19-20-16(23-15)14-9(3)18-10(4)22-14/h5-8H,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149588

(CHEMBL3770302)Show InChI InChI=1S/C15H15ClN4OS/c1-9(2)21-13-5-4-10(6-11(13)16)14-18-19-15(22-14)12-7-17-8-20(12)3/h4-9H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149589

(CHEMBL3770772)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-c1cnn(CC(O)=O)c1 Show InChI InChI=1S/C16H15ClN4O3S/c1-9(2)24-13-4-3-10(5-12(13)17)15-19-20-16(25-15)11-6-18-21(7-11)8-14(22)23/h3-7,9H,8H2,1-2H3,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149555

(CHEMBL3770278)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc(C)c(CC(O)=O)c1C Show InChI InChI=1S/C18H19ClN4O3S/c1-9(2)26-15-6-5-12(7-14(15)19)17-20-21-18(27-17)23-11(4)13(8-16(24)25)10(3)22-23/h5-7,9H,8H2,1-4H3,(H,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149556

(CHEMBL3770208)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc(C)c(CCC(O)=O)c1C Show InChI InChI=1S/C19H21ClN4O3S/c1-10(2)27-16-7-5-13(9-15(16)20)18-21-22-19(28-18)24-12(4)14(11(3)23-24)6-8-17(25)26/h5,7,9-10H,6,8H2,1-4H3,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149590

(CHEMBL3769703)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-c1cnn(CCC(O)=O)c1C Show InChI InChI=1S/C18H19ClN4O3S/c1-10(2)26-15-5-4-12(8-14(15)19)17-21-22-18(27-17)13-9-20-23(11(13)3)7-6-16(24)25/h4-5,8-10H,6-7H2,1-3H3,(H,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149591

(CHEMBL3769524)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-c1cnn(CCN2CCOCC2)c1 Show InChI InChI=1S/C20H24ClN5O2S/c1-14(2)28-18-4-3-15(11-17(18)21)19-23-24-20(29-19)16-12-22-26(13-16)6-5-25-7-9-27-10-8-25/h3-4,11-14H,5-10H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149557
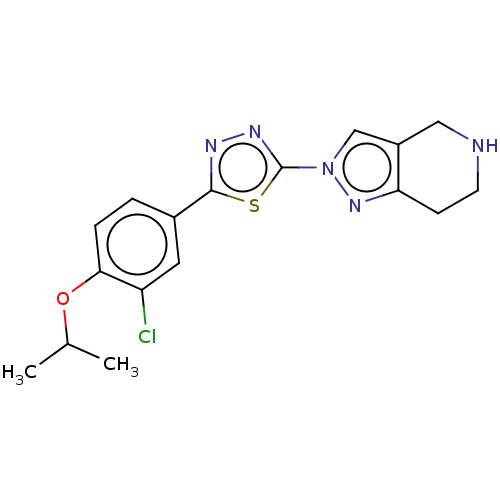
(CHEMBL3769716)Show InChI InChI=1S/C17H18ClN5OS/c1-10(2)24-15-4-3-11(7-13(15)18)16-20-21-17(25-16)23-9-12-8-19-6-5-14(12)22-23/h3-4,7,9-10,19H,5-6,8H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149558
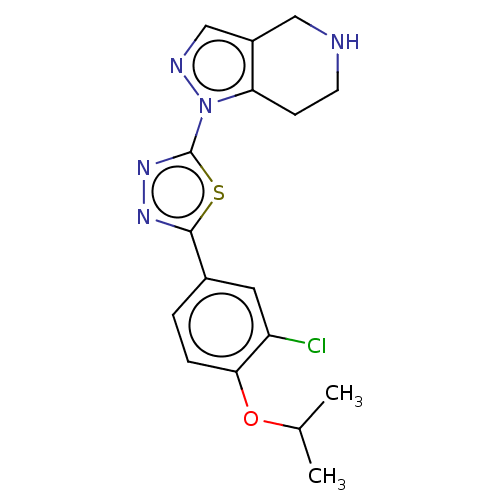
(CHEMBL3771116)Show InChI InChI=1S/C17H18ClN5OS/c1-10(2)24-15-4-3-11(7-13(15)18)16-21-22-17(25-16)23-14-5-6-19-8-12(14)9-20-23/h3-4,7,9-10,19H,5-6,8H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149559
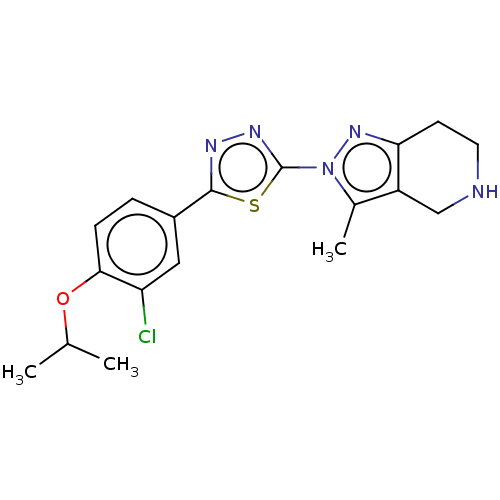
(CHEMBL3769705)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCNCc2c1C Show InChI InChI=1S/C18H20ClN5OS/c1-10(2)25-16-5-4-12(8-14(16)19)17-21-22-18(26-17)24-11(3)13-9-20-7-6-15(13)23-24/h4-5,8,10,20H,6-7,9H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50149560
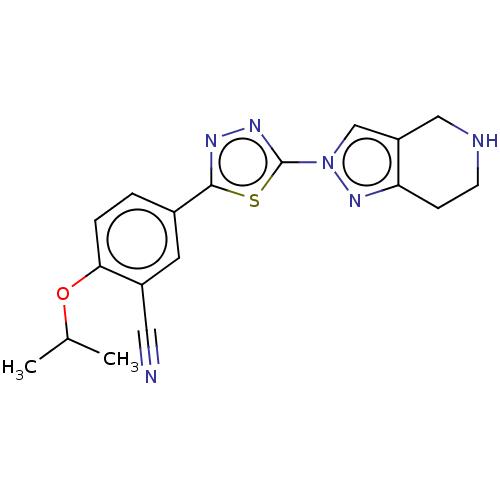
(CHEMBL3770025)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nnc(s1)-n1cc2CNCCc2n1 Show InChI InChI=1S/C18H18N6OS/c1-11(2)25-16-4-3-12(7-13(16)8-19)17-21-22-18(26-17)24-10-14-9-20-6-5-15(14)23-24/h3-4,7,10-11,20H,5-6,9H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor expressed in RBL cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data