 Found 173 hits with Last Name = 'dowell' and Initial = 'sj'
Found 173 hits with Last Name = 'dowell' and Initial = 'sj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50149592
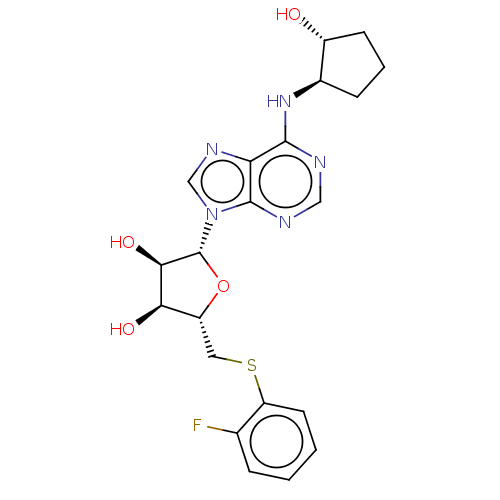
(CVT-3619 | GS-9667)Show SMILES O[C@@H]1CCC[C@H]1Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSc2ccccc2F)[C@@H](O)[C@H]1O Show InChI InChI=1S/C21H24FN5O4S/c22-11-4-1-2-7-15(11)32-8-14-17(29)18(30)21(31-14)27-10-25-16-19(23-9-24-20(16)27)26-12-5-3-6-13(12)28/h1-2,4,7,9-10,12-14,17-18,21,28-30H,3,5-6,8H2,(H,23,24,26)/t12-,13-,14-,17-,18-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Displacement of [3H]CPX from human Adenosine A1 receptor expressed in DDT1MF-2 cells |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50149592

(CVT-3619 | GS-9667)Show SMILES O[C@@H]1CCC[C@H]1Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSc2ccccc2F)[C@@H](O)[C@H]1O Show InChI InChI=1S/C21H24FN5O4S/c22-11-4-1-2-7-15(11)32-8-14-17(29)18(30)21(31-14)27-10-25-16-19(23-9-24-20(16)27)26-12-5-3-6-13(12)28/h1-2,4,7,9-10,12-14,17-18,21,28-30H,3,5-6,8H2,(H,23,24,26)/t12-,13-,14-,17-,18-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Displacement of [3H]CPX from human Adenosine A1 receptor expressed in CHO cells |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50149598
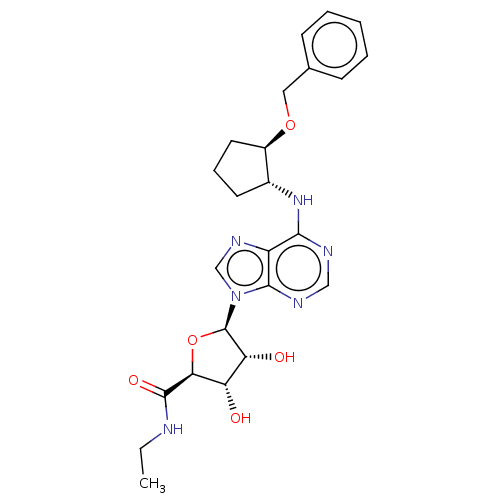
(CHEMBL3770679)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCC[C@H]3OCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C24H30N6O5/c1-2-25-23(33)20-18(31)19(32)24(35-20)30-13-28-17-21(26-12-27-22(17)30)29-15-9-6-10-16(15)34-11-14-7-4-3-5-8-14/h3-5,7-8,12-13,15-16,18-20,24,31-32H,2,6,9-11H2,1H3,(H,25,33)(H,26,27,29)/t15-,16-,18+,19-,20+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0295 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21220
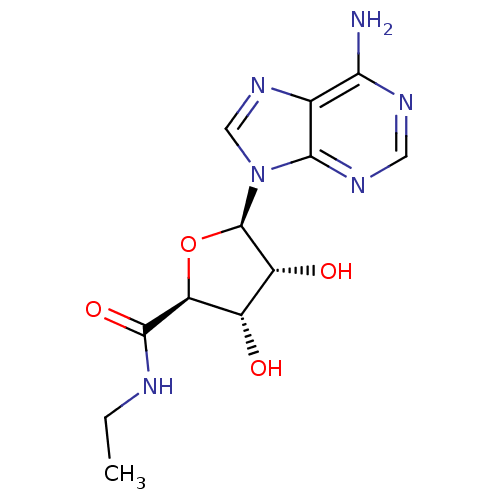
((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50149597
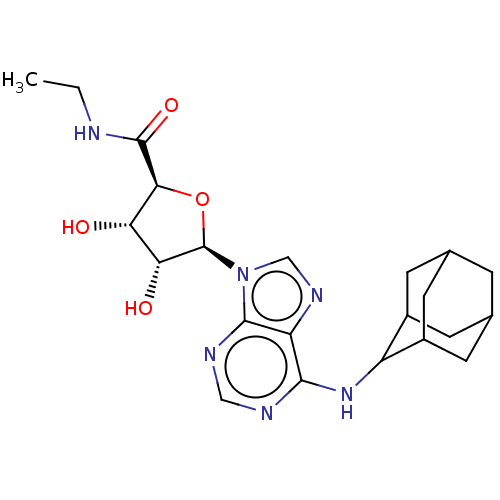
(CHEMBL3771184)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3C4CC5CC(C4)CC3C5)ncnc12 |r,wU:7.12,5.4,wD:8.8,10.11,TLB:24:23:19.20.18:27,17:18:22.23.25:27,THB:18:19:22:25.26.27,18:26:22:19.20.24,24:19:22.23.25:27,(15.69,-2.49,;14.45,-2.59,;13.56,-1.31,;12.01,-1.44,;11.48,-2.57,;11.12,-.17,;9.56,-.13,;9.14,1.36,;10.39,2.25,;10.41,3.49,;11.63,1.31,;12.82,1.66,;7.63,1.76,;7.09,3.19,;5.53,3.12,;5.13,1.62,;3.73,.92,;2.42,1.78,;1.02,1.07,;1.12,-.36,;-.4,-.89,;-1.87,-.71,;-2.28,-2.13,;-1.05,-1.34,;.37,-1.61,;-1.05,.45,;-.29,1.71,;-2.05,.73,;3.65,-.65,;4.96,-1.49,;6.37,-.78,;6.43,.78,)| Show InChI InChI=1S/C22H30N6O4/c1-2-23-21(31)18-16(29)17(30)22(32-18)28-9-26-15-19(24-8-25-20(15)28)27-14-12-4-10-3-11(6-12)7-13(14)5-10/h8-14,16-18,22,29-30H,2-7H2,1H3,(H,23,31)(H,24,25,27)/t10?,11?,12?,13?,14?,16-,17+,18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50085658

((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50149594

(CHEMBL3771208)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCC[C@H]3OCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C22H27N5O5/c28-9-16-18(29)19(30)22(32-16)27-12-25-17-20(23-11-24-21(17)27)26-14-7-4-8-15(14)31-10-13-5-2-1-3-6-13/h1-3,5-6,11-12,14-16,18-19,22,28-30H,4,7-10H2,(H,23,24,26)/t14-,15-,16-,18-,19-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50149599
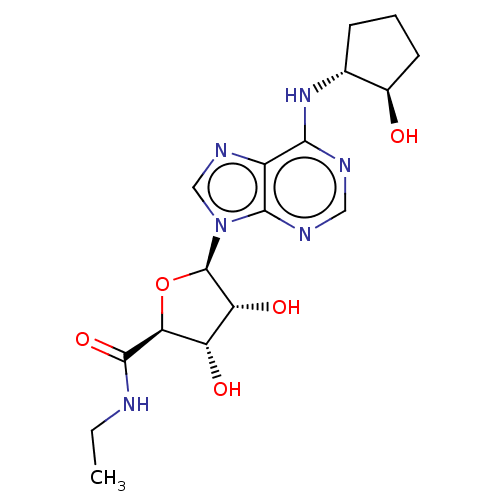
(CHEMBL3770310)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCC[C@H]3O)ncnc12 |r| Show InChI InChI=1S/C17H24N6O5/c1-2-18-16(27)13-11(25)12(26)17(28-13)23-7-21-10-14(19-6-20-15(10)23)22-8-4-3-5-9(8)24/h6-9,11-13,17,24-26H,2-5H2,1H3,(H,18,27)(H,19,20,22)/t8-,9-,11+,12-,13+,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50149595

(CHEMBL3770664)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC34CC5CC(CC(C5)C3)C4)ncnc12 |r,TLB:25:20:27:24.26.23,25:24:27:21.20.19,THB:19:18:21.20.25:23,19:20:18.27.26:23,17:18:21.20.25:23| Show InChI InChI=1S/C22H30N6O4/c1-2-23-20(31)17-15(29)16(30)21(32-17)28-10-26-14-18(24-9-25-19(14)28)27-22-6-11-3-12(7-22)5-13(4-11)8-22/h9-13,15-17,21,29-30H,2-8H2,1H3,(H,23,31)(H,24,25,27)/t11?,12?,13?,15-,16+,17-,21+,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080399
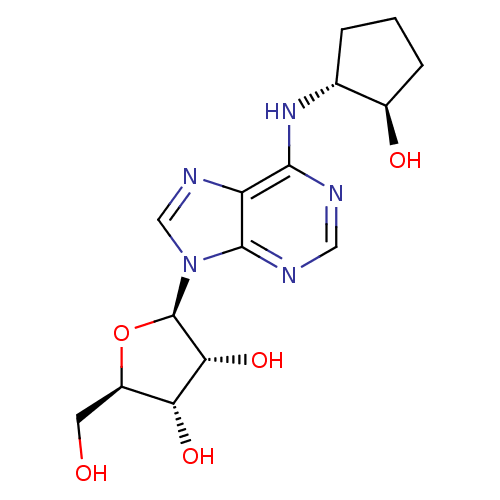
((2R,3R,4S,5R)-2-[6-((1R,2R)-2-Hydroxy-cyclopentyla...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCC[C@H]3O)ncnc12 Show InChI InChI=1S/C15H21N5O5/c21-4-9-11(23)12(24)15(25-9)20-6-18-10-13(16-5-17-14(10)20)19-7-2-1-3-8(7)22/h5-9,11-12,15,21-24H,1-4H2,(H,16,17,19)/t7-,8-,9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50149599

(CHEMBL3770310)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCC[C@H]3O)ncnc12 |r| Show InChI InChI=1S/C17H24N6O5/c1-2-18-16(27)13-11(25)12(26)17(28-13)23-7-21-10-14(19-6-20-15(10)23)22-8-4-3-5-9(8)24/h6-9,11-13,17,24-26H,2-5H2,1H3,(H,18,27)(H,19,20,22)/t8-,9-,11+,12-,13+,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50085658

((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50149593
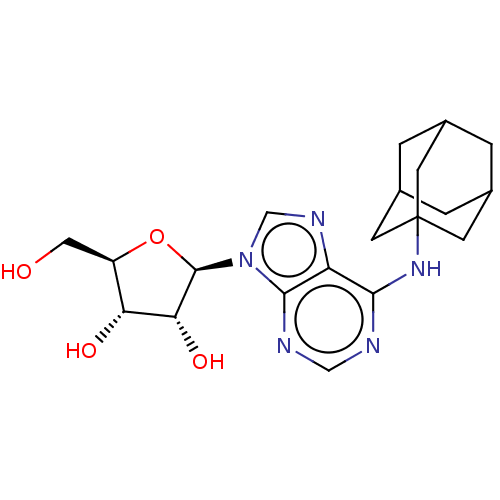
(CHEMBL3771290)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC34CC5CC(CC(C5)C3)C4)ncnc12 |r,TLB:22:17:24:21.23.20,22:21:24:18.17.16,THB:16:15:18.17.22:20,16:17:15.24.23:20,14:15:18.17.22:20| Show InChI InChI=1S/C20H27N5O4/c26-7-13-15(27)16(28)19(29-13)25-9-23-14-17(21-8-22-18(14)25)24-20-4-10-1-11(5-20)3-12(2-10)6-20/h8-13,15-16,19,26-28H,1-7H2,(H,21,22,24)/t10?,11?,12?,13-,15-,16-,19-,20?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50080399

((2R,3R,4S,5R)-2-[6-((1R,2R)-2-Hydroxy-cyclopentyla...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCC[C@H]3O)ncnc12 Show InChI InChI=1S/C15H21N5O5/c21-4-9-11(23)12(24)15(25-9)20-6-18-10-13(16-5-17-14(10)20)19-7-2-1-3-8(7)22/h5-9,11-12,15,21-24H,1-4H2,(H,16,17,19)/t7-,8-,9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50149596

(CHEMBL3769641)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC34CC5CC(CC(O)(C5)C3)C4)ncnc12 |r,TLB:25:24:21:18.28.19,17:18:21.20.26:23,THB:27:18:21:26.24.23,27:24:21:18.28.19,19:18:21.20.26:23,19:20:18.28.27:23,17:18:21:26.24.23| Show InChI InChI=1S/C22H30N6O5/c1-2-23-19(31)16-14(29)15(30)20(33-16)28-10-26-13-17(24-9-25-18(13)28)27-21-4-11-3-12(5-21)7-22(32,6-11)8-21/h9-12,14-16,20,29-30,32H,2-8H2,1H3,(H,23,31)(H,24,25,27)/t11?,12?,14-,15+,16-,20+,21?,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50149596

(CHEMBL3769641)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC34CC5CC(CC(O)(C5)C3)C4)ncnc12 |r,TLB:25:24:21:18.28.19,17:18:21.20.26:23,THB:27:18:21:26.24.23,27:24:21:18.28.19,19:18:21.20.26:23,19:20:18.28.27:23,17:18:21:26.24.23| Show InChI InChI=1S/C22H30N6O5/c1-2-23-19(31)16-14(29)15(30)20(33-16)28-10-26-13-17(24-9-25-18(13)28)27-21-4-11-3-12(5-21)7-22(32,6-11)8-21/h9-12,14-16,20,29-30,32H,2-8H2,1H3,(H,23,31)(H,24,25,27)/t11?,12?,14-,15+,16-,20+,21?,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50149598

(CHEMBL3770679)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCC[C@H]3OCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C24H30N6O5/c1-2-25-23(33)20-18(31)19(32)24(35-20)30-13-28-17-21(26-12-27-22(17)30)29-15-9-6-10-16(15)34-11-14-7-4-3-5-8-14/h3-5,7-8,12-13,15-16,18-20,24,31-32H,2,6,9-11H2,1H3,(H,25,33)(H,26,27,29)/t15-,16-,18+,19-,20+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 269 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick
Curated by ChEMBL
| Assay Description
Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... |
J Med Chem 59: 947-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01402
BindingDB Entry DOI: 10.7270/Q2FX7CBN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419206
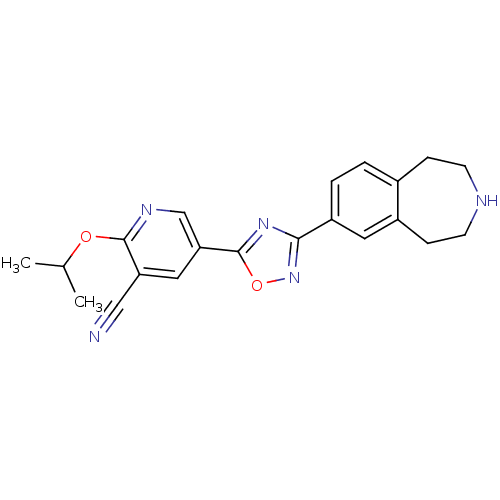
(CHEMBL1836170)Show SMILES CC(C)Oc1ncc(cc1C#N)-c1nc(no1)-c1ccc2CCNCCc2c1 Show InChI InChI=1S/C21H21N5O2/c1-13(2)27-20-17(11-22)10-18(12-24-20)21-25-19(26-28-21)16-4-3-14-5-7-23-8-6-15(14)9-16/h3-4,9-10,12-13,23H,5-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50354138
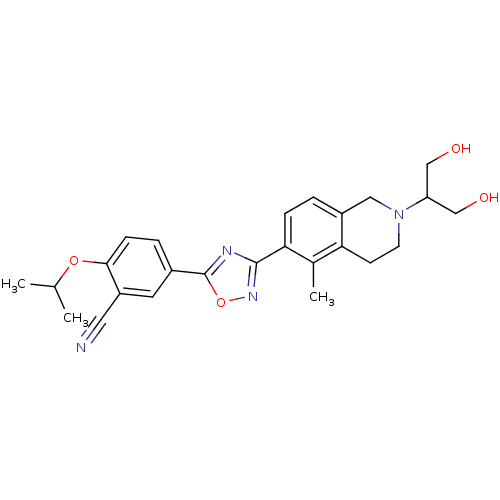
(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419207
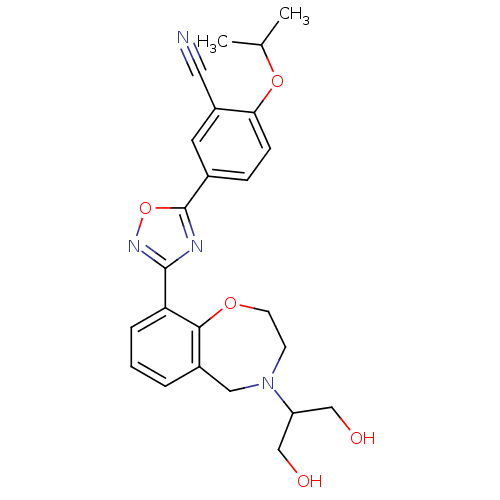
(CHEMBL1836212)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1cccc2CN(CCOc12)C(CO)CO Show InChI InChI=1S/C24H26N4O5/c1-15(2)32-21-7-6-16(10-18(21)11-25)24-26-23(27-33-24)20-5-3-4-17-12-28(19(13-29)14-30)8-9-31-22(17)20/h3-7,10,15,19,29-30H,8-9,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using vivid green as substrate |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419201

(CHEMBL1836171)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CC(=O)N[C@@H](C)CO)CCc2c1C |r| Show InChI InChI=1S/C27H31N5O4/c1-16(2)35-24-8-6-19(11-21(24)12-28)27-30-26(31-36-27)23-7-5-20-13-32(10-9-22(20)18(23)4)14-25(34)29-17(3)15-33/h5-8,11,16-17,33H,9-10,13-15H2,1-4H3,(H,29,34)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419204

(CHEMBL1836213)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1cnc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C24H27N5O4/c1-14(2)32-22-5-4-16(8-17(22)9-25)24-27-23(28-33-24)20-10-26-21-11-29(18(12-30)13-31)7-6-19(21)15(20)3/h4-5,8,10,14,18,30-31H,6-7,11-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using vivid red as substrate |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419205
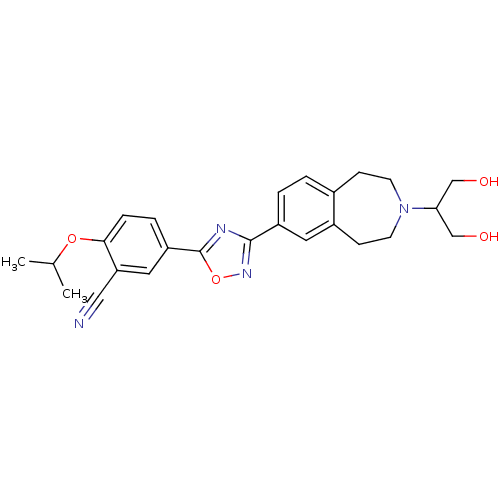
(CHEMBL1836172)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CCN(CCc2c1)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-16(2)32-23-6-5-20(12-21(23)13-26)25-27-24(28-33-25)19-4-3-17-7-9-29(22(14-30)15-31)10-8-18(17)11-19/h3-6,11-12,16,22,30-31H,7-10,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50419203
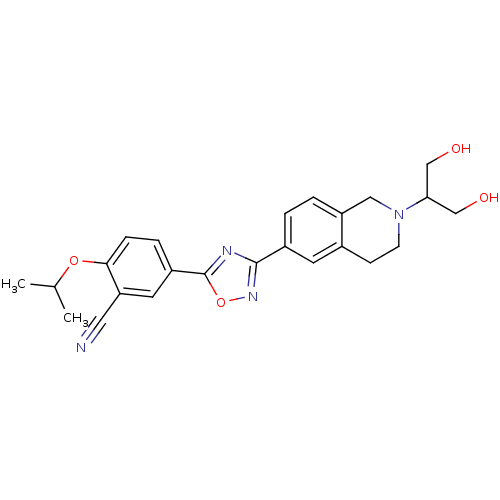
(CHEMBL1836214)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1)C(CO)CO Show InChI InChI=1S/C24H26N4O4/c1-15(2)31-22-6-5-18(10-20(22)11-25)24-26-23(27-32-24)17-3-4-19-12-28(21(13-29)14-30)8-7-16(19)9-17/h3-6,9-10,15,21,29-30H,7-8,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50149566
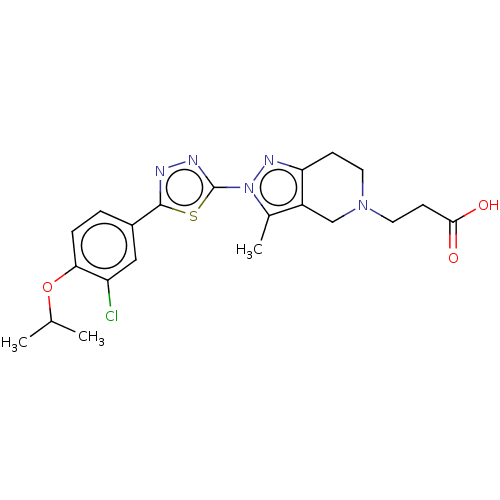
(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by VG metabolism assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) coexpressed in Escherichia coli with human NADPH reductase using 3-butyryl-7-methoxycoumarin as substrate afte... |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by VR metabolism assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) coexpressed in Escherichia coli with human NADPH reductase using 7-methoxy-4-trifluoromethylcoumarin-3-acetic a... |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50354138

(CHEMBL1836215)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO Show InChI InChI=1S/C25H28N4O4/c1-15(2)32-23-7-5-17(10-19(23)11-26)25-27-24(28-33-25)22-6-4-18-12-29(20(13-30)14-31)9-8-21(18)16(22)3/h4-7,10,15,20,30-31H,8-9,12-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 54: 6724-33 (2011)
Article DOI: 10.1021/jm200609t
BindingDB Entry DOI: 10.7270/Q27P8ZSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) coexpressed in Escherichia coli with human NADPH reductase using 4-methylaminomethyl-7-methoyxycoumarin as subs... |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) coexpressed in Escherichia coli with human NADPH reductase using ethoxyresorufin as substrate after 10 mins by ... |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50149566

(CHEMBL3769933)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-n1nc2CCN(CCC(O)=O)Cc2c1C Show InChI InChI=1S/C21H24ClN5O3S/c1-12(2)30-18-5-4-14(10-16(18)22)20-23-24-21(31-20)27-13(3)15-11-26(9-7-19(28)29)8-6-17(15)25-27/h4-5,10,12H,6-9,11H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149581
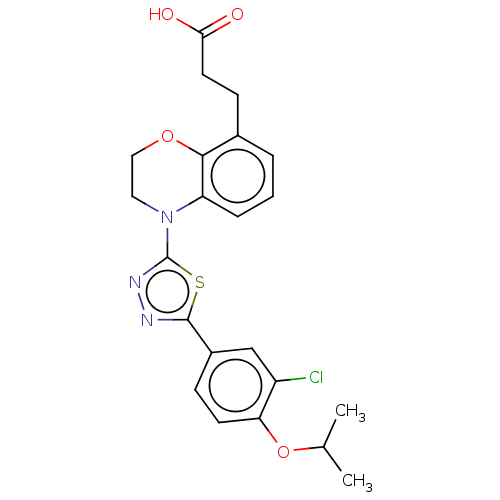
(CHEMBL3771149)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)N1CCOc2c(CCC(O)=O)cccc12 Show InChI InChI=1S/C22H22ClN3O4S/c1-13(2)30-18-8-6-15(12-16(18)23)21-24-25-22(31-21)26-10-11-29-20-14(7-9-19(27)28)4-3-5-17(20)26/h3-6,8,12-13H,7,9-11H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149582

(CHEMBL3770821)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)N1CCc2cc(CCC(O)=O)ccc12 Show InChI InChI=1S/C22H22ClN3O3S/c1-13(2)29-19-7-5-16(12-17(19)23)21-24-25-22(30-21)26-10-9-15-11-14(3-6-18(15)26)4-8-20(27)28/h3,5-7,11-13H,4,8-10H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149572
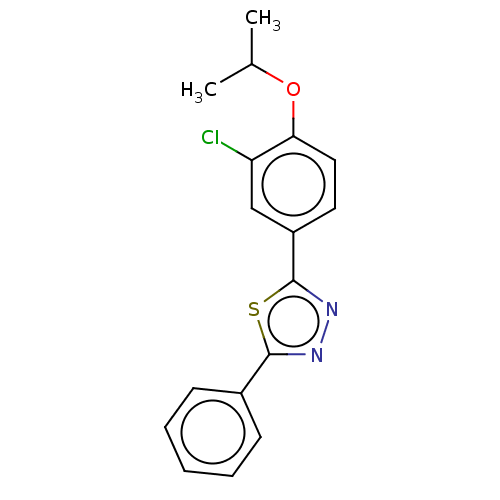
(CHEMBL3771092)Show InChI InChI=1S/C17H15ClN2OS/c1-11(2)21-15-9-8-13(10-14(15)18)17-20-19-16(22-17)12-6-4-3-5-7-12/h3-11H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149573
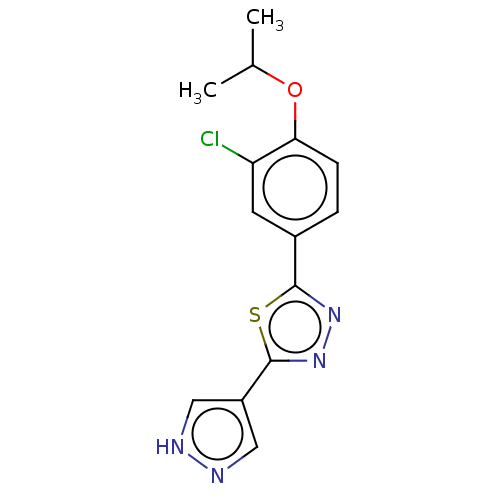
(CHEMBL3769742)Show InChI InChI=1S/C14H13ClN4OS/c1-8(2)20-12-4-3-9(5-11(12)15)13-18-19-14(21-13)10-6-16-17-7-10/h3-8H,1-2H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149583
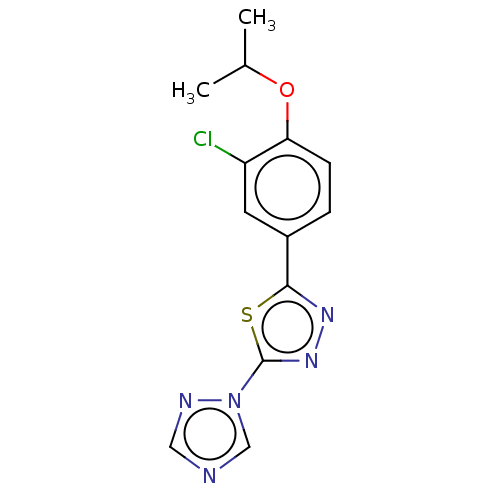
(CHEMBL3770492)Show InChI InChI=1S/C13H12ClN5OS/c1-8(2)20-11-4-3-9(5-10(11)14)12-17-18-13(21-12)19-7-15-6-16-19/h3-8H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149584
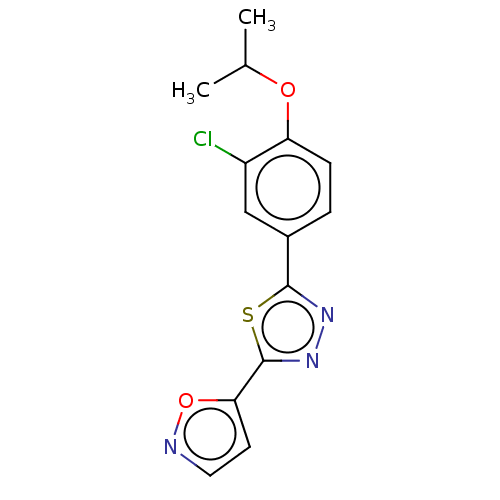
(CHEMBL3770423)Show InChI InChI=1S/C14H12ClN3O2S/c1-8(2)19-11-4-3-9(7-10(11)15)13-17-18-14(21-13)12-5-6-16-20-12/h3-8H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149585
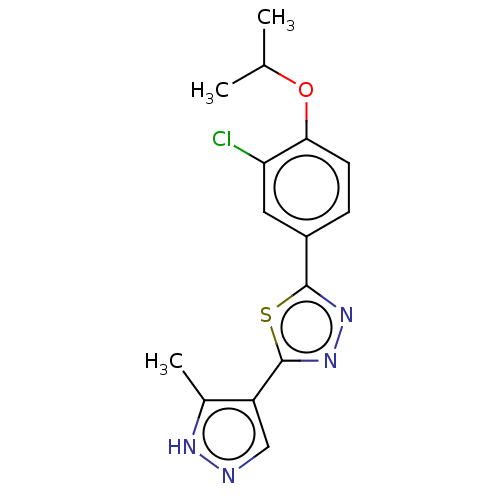
(CHEMBL3770352)Show InChI InChI=1S/C15H15ClN4OS/c1-8(2)21-13-5-4-10(6-12(13)16)14-19-20-15(22-14)11-7-17-18-9(11)3/h4-8H,1-3H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149586
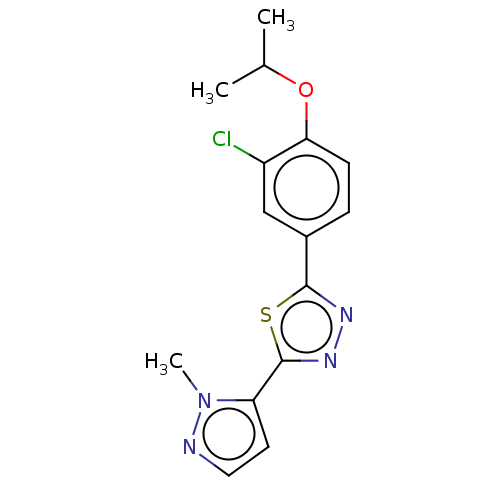
(CHEMBL3770390)Show InChI InChI=1S/C15H15ClN4OS/c1-9(2)21-13-5-4-10(8-11(13)16)14-18-19-15(22-14)12-6-7-17-20(12)3/h4-9H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149587

(CHEMBL3770587)Show InChI InChI=1S/C16H16ClN3OS2/c1-8(2)21-13-6-5-11(7-12(13)17)15-19-20-16(23-15)14-9(3)18-10(4)22-14/h5-8H,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149588
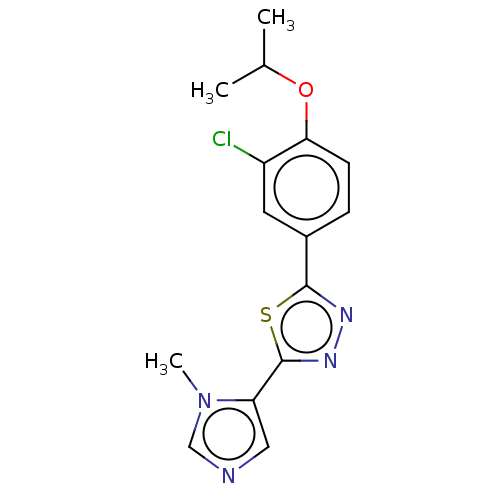
(CHEMBL3770302)Show InChI InChI=1S/C15H15ClN4OS/c1-9(2)21-13-5-4-10(6-11(13)16)14-18-19-15(22-14)12-7-17-8-20(12)3/h4-9H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50149589

(CHEMBL3770772)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nnc(s1)-c1cnn(CC(O)=O)c1 Show InChI InChI=1S/C16H15ClN4O3S/c1-9(2)24-13-4-3-10(5-12(13)17)15-19-20-16(25-15)11-6-18-21(7-11)8-14(22)23/h3-7,9H,8H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in RH7777 cells by [35S]GTP-gammaS accumulation assay |
J Med Chem 59: 1003-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01512
BindingDB Entry DOI: 10.7270/Q2KP842C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data