

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50054198 (CHEMBL3310888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graph | Bioorg Med Chem 22: 3957-70 (2014) Article DOI: 10.1016/j.bmc.2014.06.007 BindingDB Entry DOI: 10.7270/Q28P625B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50054196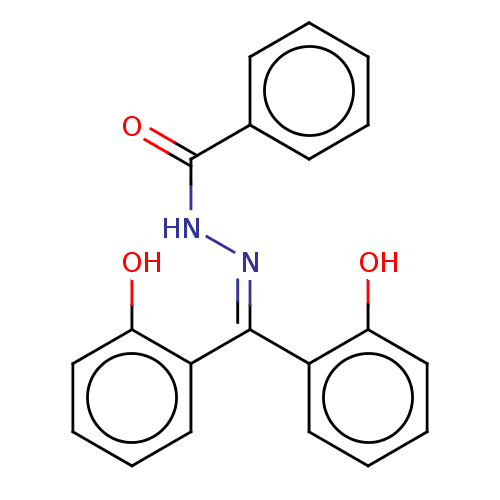 (CHEMBL3310887) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graph | Bioorg Med Chem 22: 3957-70 (2014) Article DOI: 10.1016/j.bmc.2014.06.007 BindingDB Entry DOI: 10.7270/Q28P625B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50043758 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-hexylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50054194 (CHEMBL3310886) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graph | Bioorg Med Chem 22: 3957-70 (2014) Article DOI: 10.1016/j.bmc.2014.06.007 BindingDB Entry DOI: 10.7270/Q28P625B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50295556 (C6H5-SO2-Glu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-G...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by 1-chloro-2,4-dinitrobenzene competitive assay | Eur J Med Chem 44: 2009-16 (2009) Article DOI: 10.1016/j.ejmech.2008.10.009 BindingDB Entry DOI: 10.7270/Q2S182J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50295554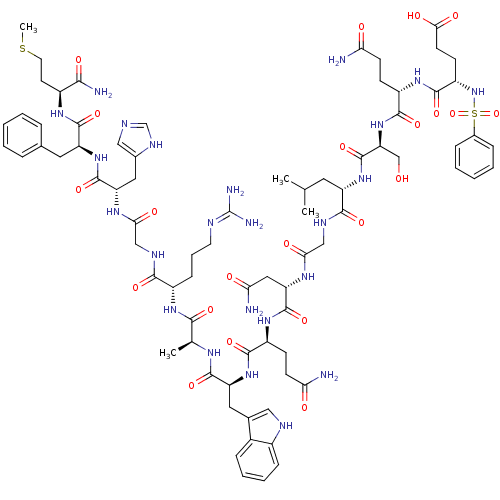 (C6H5-SO2-Glu-Gln-Ser-Leu-Gly-Asn-Gln-Trp-Ala-Arg-G...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by 1-chloro-2,4-dinitrobenzene competitive assay | Eur J Med Chem 44: 2009-16 (2009) Article DOI: 10.1016/j.ejmech.2008.10.009 BindingDB Entry DOI: 10.7270/Q2S182J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50295555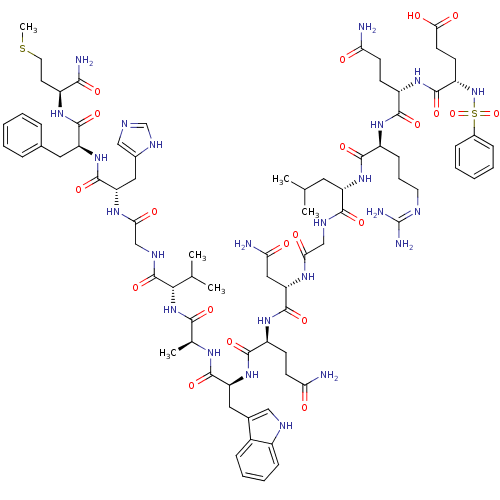 (C6H5-SO2-Glu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-G...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by 1-chloro-2,4-dinitrobenzene competitive assay | Eur J Med Chem 44: 2009-16 (2009) Article DOI: 10.1016/j.ejmech.2008.10.009 BindingDB Entry DOI: 10.7270/Q2S182J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50054205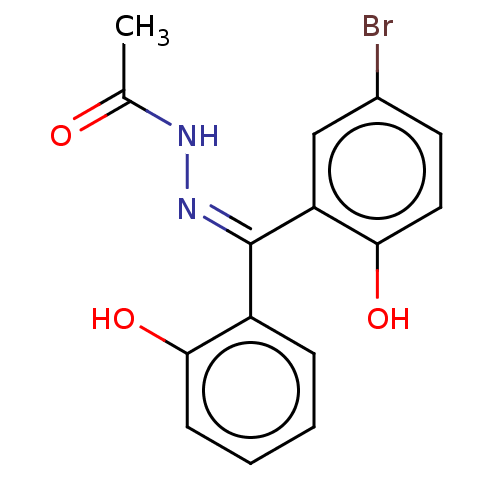 (CHEMBL3310889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graph | Bioorg Med Chem 22: 3957-70 (2014) Article DOI: 10.1016/j.bmc.2014.06.007 BindingDB Entry DOI: 10.7270/Q28P625B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50043764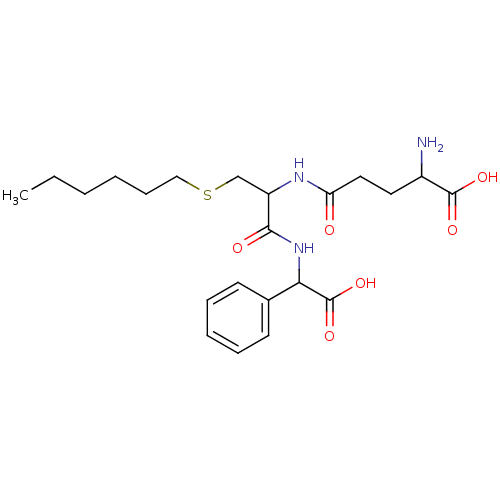 (2-Amino-4-{1-[(carboxy-phenyl-methyl)-carbamoyl]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50295556 (C6H5-SO2-Glu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-G...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by glutathione competitive assay | Eur J Med Chem 44: 2009-16 (2009) Article DOI: 10.1016/j.ejmech.2008.10.009 BindingDB Entry DOI: 10.7270/Q2S182J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50295554 (C6H5-SO2-Glu-Gln-Ser-Leu-Gly-Asn-Gln-Trp-Ala-Arg-G...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by glutathione competitive assay | Eur J Med Chem 44: 2009-16 (2009) Article DOI: 10.1016/j.ejmech.2008.10.009 BindingDB Entry DOI: 10.7270/Q2S182J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50295555 (C6H5-SO2-Glu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-G...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by glutathione competitive assay | Eur J Med Chem 44: 2009-16 (2009) Article DOI: 10.1016/j.ejmech.2008.10.009 BindingDB Entry DOI: 10.7270/Q2S182J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50043760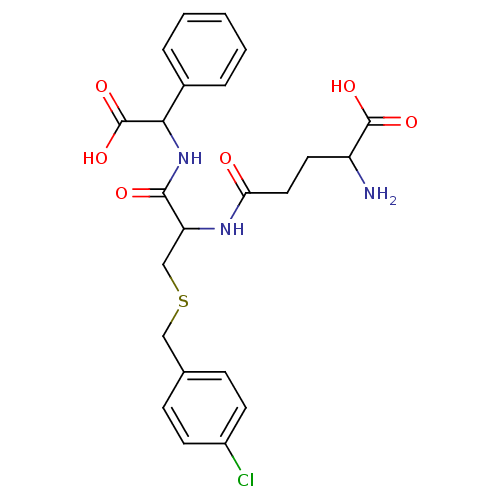 (2-Amino-4-[1-[(carboxy-phenyl-methyl)-carbamoyl]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50043762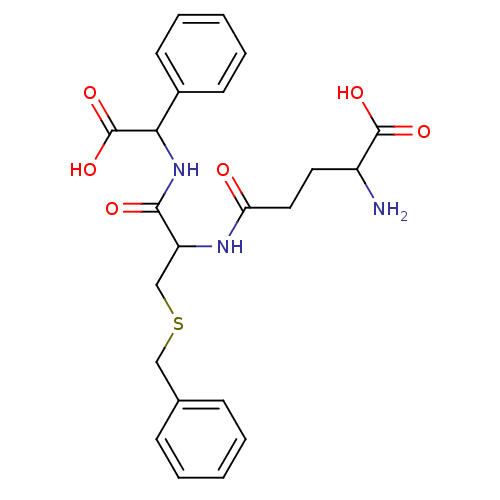 (2-Amino-4-{2-benzylsulfanyl-1-[(carboxy-phenyl-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50043761 (2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-hexylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50043763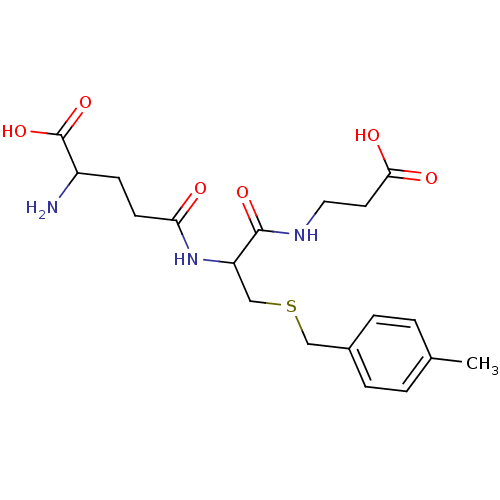 (2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-(4-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50395590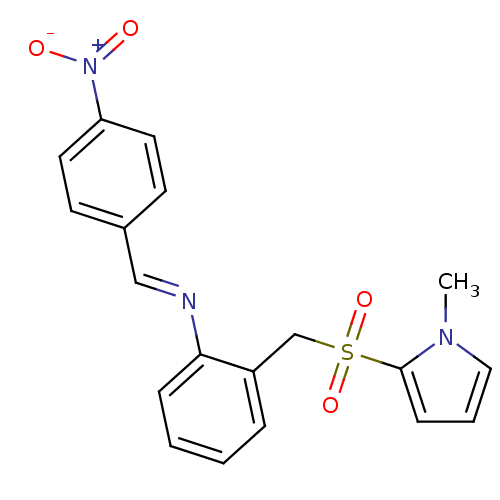 (CHEMBL2165141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human GSTA1-1 expressed in Escherichia coli BL21 (DE3) using CDNB as substrate | J Med Chem 55: 6802-13 (2012) Article DOI: 10.1021/jm300385f BindingDB Entry DOI: 10.7270/Q2DN4654 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50395591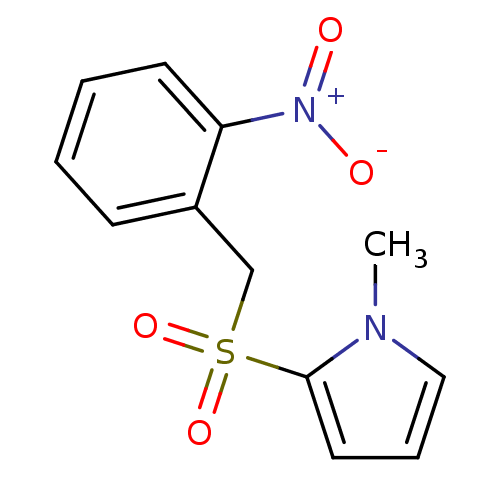 (CHEMBL2165146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agricultural University of Athens Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human GSTA1-1 expressed in Escherichia coli BL21 (DE3) using CDNB as substrate | J Med Chem 55: 6802-13 (2012) Article DOI: 10.1021/jm300385f BindingDB Entry DOI: 10.7270/Q2DN4654 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50043759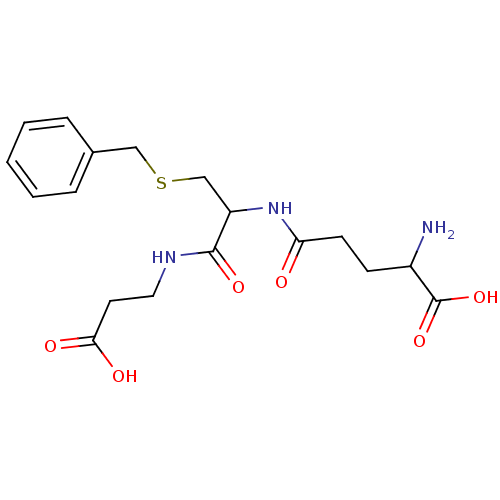 (2-Amino-4-[2-benzylsulfanyl-1-(2-carboxy-ethylcarb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218770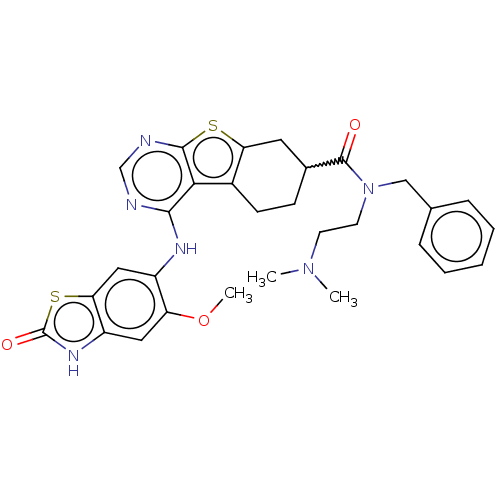 (US9296757, 92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218771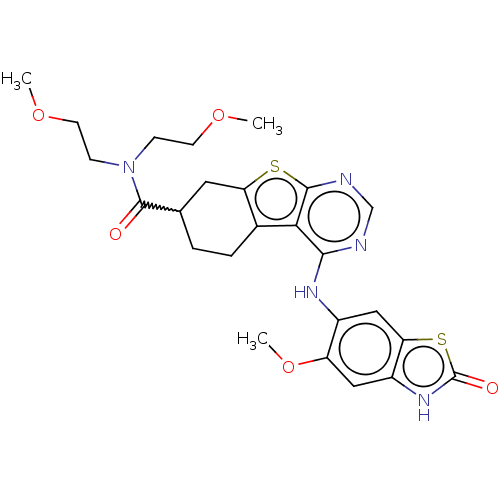 (US9296757, 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218768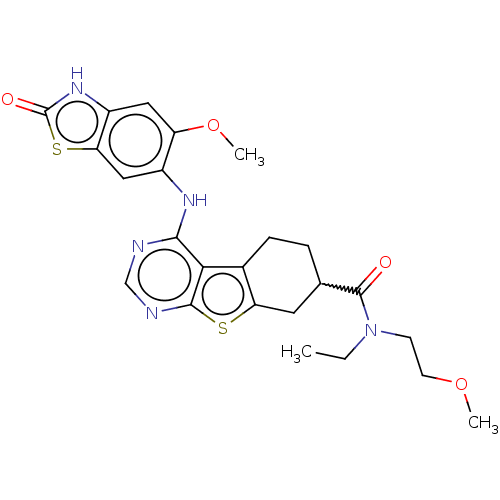 (US9296757, 90) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218766 (US9296757, 88) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218767 (US9296757, 89) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218765 (US9296757, 87) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218764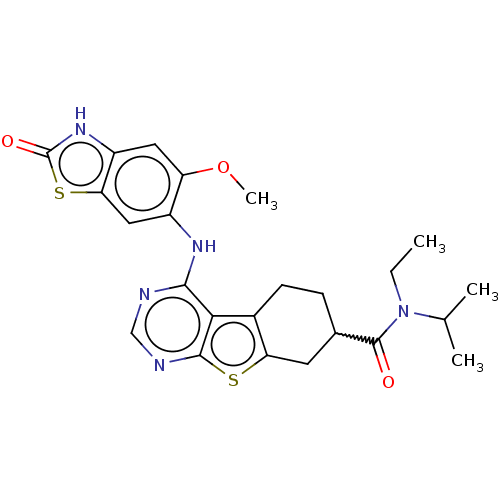 (US9296757, 86) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218763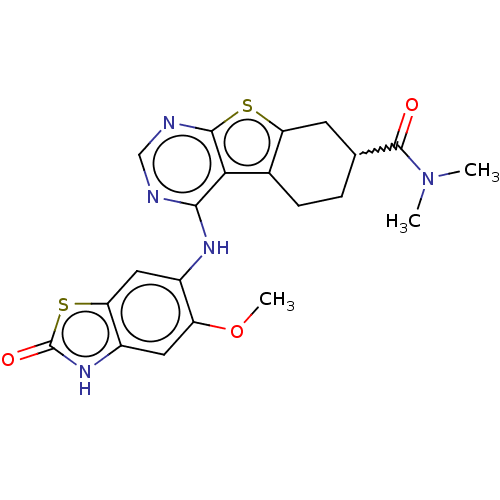 (US9296757, 85) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218709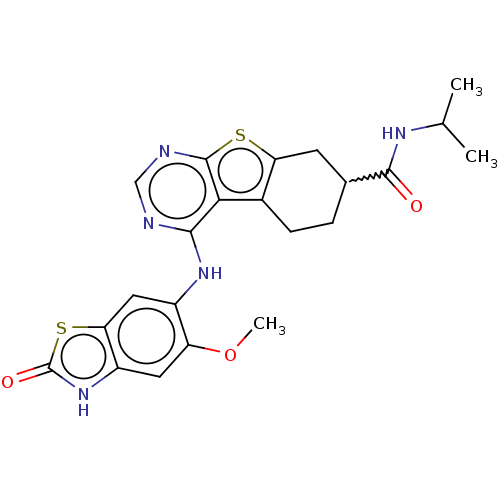 (US9296757, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214639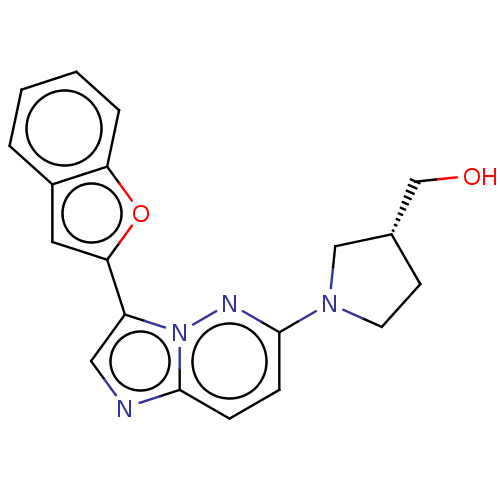 (US9284319, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214635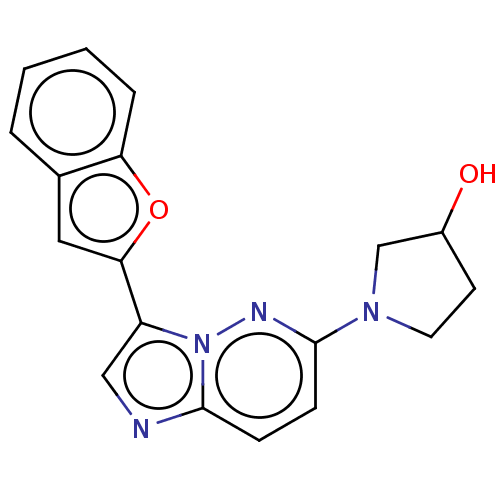 (US9284319, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214633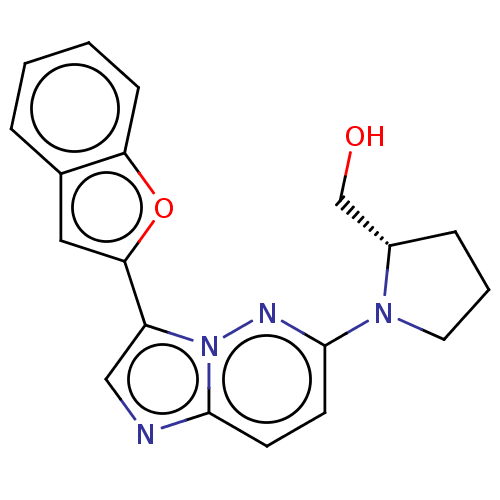 (US9284319, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218714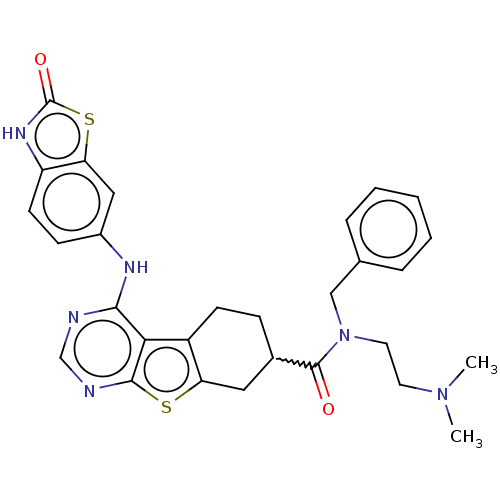 (US9296757, 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218687 (US9296757, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214649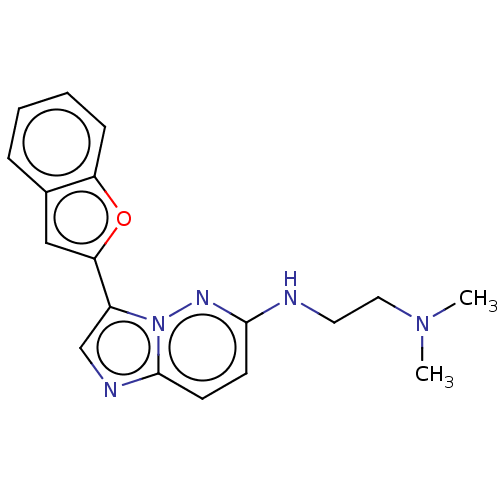 (US9284319, R7 | US9730929, Example R7 | US9783543,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218688 (US9296757, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214638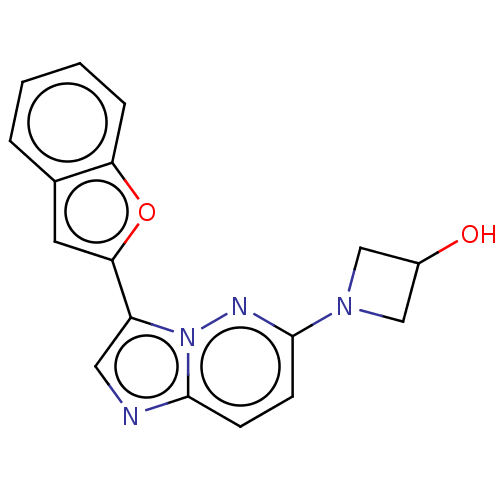 (US9284319, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214649 (US9284319, R7 | US9730929, Example R7 | US9783543,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214637 (US9284319, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50186225 (3-((3-(2-(2,3-dichloro-4-(2-methylenebutanoyl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntrix Biosytems Curated by ChEMBL | Assay Description Inhibition of GST A1-1 | Bioorg Med Chem Lett 16: 3780-3 (2006) Article DOI: 10.1016/j.bmcl.2006.04.041 BindingDB Entry DOI: 10.7270/Q28W3CXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214639 (US9284319, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1 (Homo sapiens (Human)) | BDBM50186230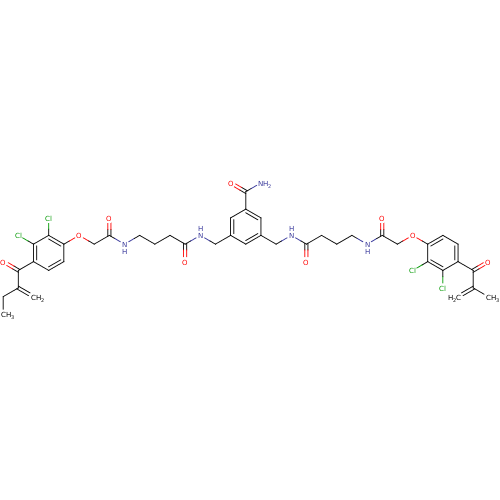 (3-((4-(2-(2,3-dichloro-4-(2-methylenebutanoyl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntrix Biosytems Curated by ChEMBL | Assay Description Inhibition of GST A1-1 | Bioorg Med Chem Lett 16: 3780-3 (2006) Article DOI: 10.1016/j.bmcl.2006.04.041 BindingDB Entry DOI: 10.7270/Q28W3CXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214640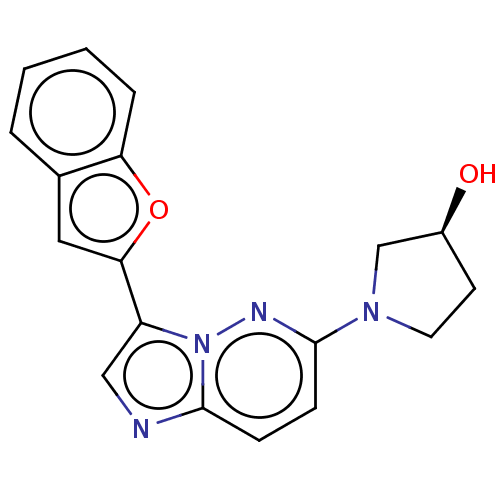 (US9284319, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214636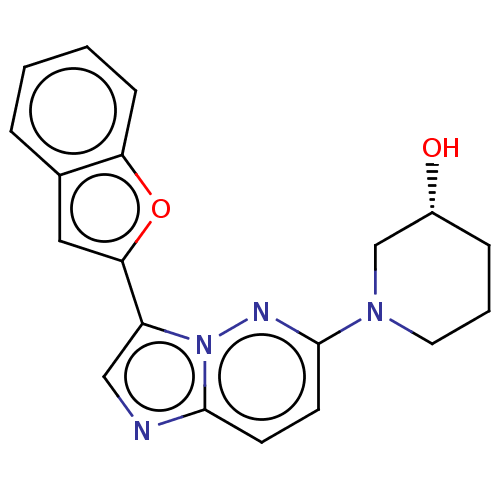 (US9284319, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214641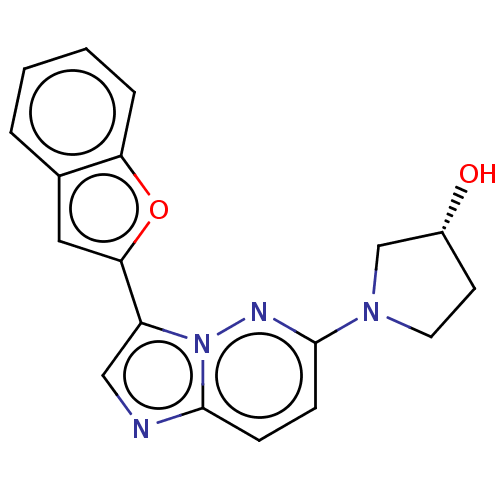 (US9284319, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218708 (US9296757, 30 | US9296757, 33) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214635 (US9284319, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214648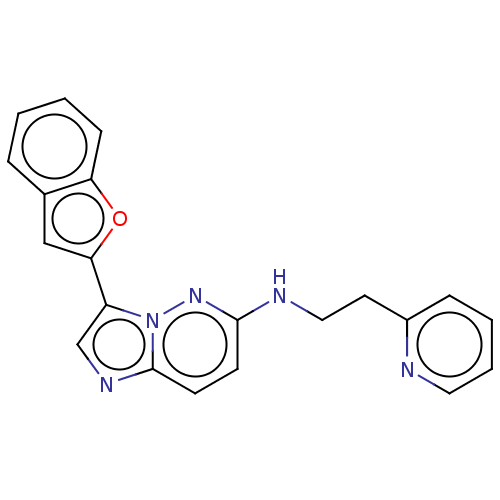 (US9284319, R6 | US9730929, Example R6 | US9783543,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214640 (US9284319, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218747 (US9296757, 69) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM214633 (US9284319, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9284319 (2016) BindingDB Entry DOI: 10.7270/Q2765D6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 174 total ) | Next | Last >> |