 Found 49 hits of affinity data for UniProtKB/TrEMBL: P80404
Found 49 hits of affinity data for UniProtKB/TrEMBL: P80404 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50024968
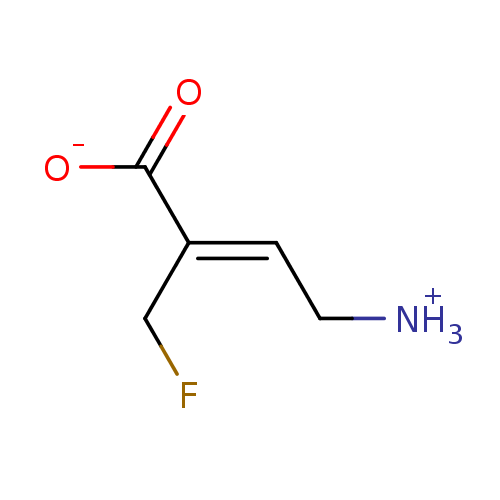
((2Z)-4-ammonio-2-(fluoromethyl)but-2-enoate | CHEM...)Show InChI InChI=1S/C5H8FNO2/c6-3-4(1-2-7)5(8)9/h1H,2-3,7H2,(H,8,9)/b4-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory effect against Gamma-amino-N-butyrate transaminase from bacteria |
J Med Chem 29: 764-70 (1986)
BindingDB Entry DOI: 10.7270/Q2668C6T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50021645
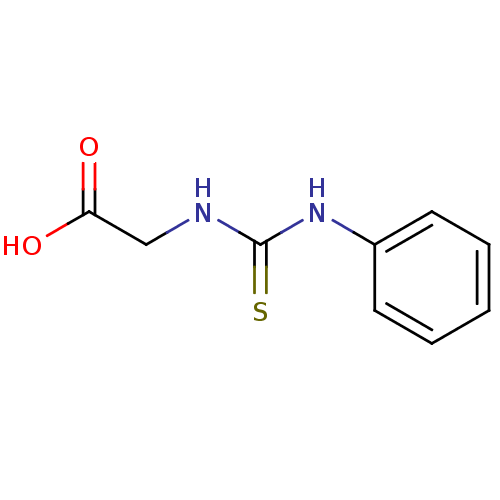
((3-Phenyl-thioureido)-acetic acid | CHEMBL311337 |...)Show InChI InChI=1S/C9H10N2O2S/c12-8(13)6-10-9(14)11-7-4-2-1-3-5-7/h1-5H,6H2,(H,12,13)(H2,10,11,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Gamma-amino-N-butyrate transaminase |
J Med Chem 30: 239-49 (1987)
BindingDB Entry DOI: 10.7270/Q2SF2V5K |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50118885

(5-Amino-cyclohex-3-enecarboxylic acid | CHEMBL1379...)Show InChI InChI=1S/C7H11NO2/c8-6-3-1-2-5(4-6)7(9)10/h1,3,5-6H,2,4,8H2,(H,9,10)/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Binding affinity against Gamma-amino-N-butyrate transaminase |
J Med Chem 45: 4531-9 (2002)
BindingDB Entry DOI: 10.7270/Q2G1605T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50118884

(2-Amino-cyclohex-3-enecarboxylic acid | CHEMBL1419...)Show InChI InChI=1S/C7H11NO2/c8-6-4-2-1-3-5(6)7(9)10/h2,4-6H,1,3,8H2,(H,9,10)/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Binding affinity against Gamma-amino-N-butyrate transaminase |
J Med Chem 45: 4531-9 (2002)
BindingDB Entry DOI: 10.7270/Q2G1605T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM323799
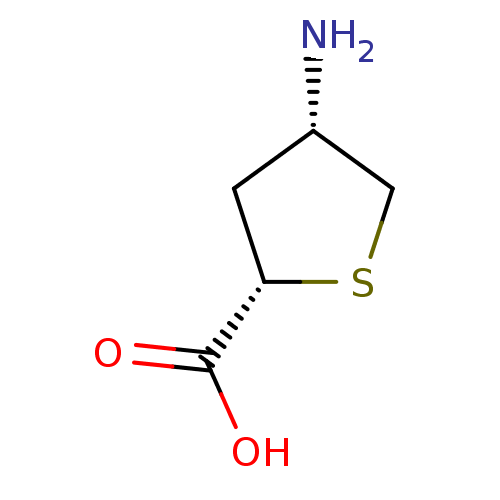
((2S,4S)-4-Aminotetrahydrothiophene-2-carboxylic ac...)Show InChI InChI=1S/C5H9NO2S/c6-3-1-4(5(7)8)9-2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... |
US Patent US10189807 (2019)
BindingDB Entry DOI: 10.7270/Q2C82CDG |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM466532

(US10800753, Compound 17)Show InChI InChI=1S/C6H11NO2S/c1-9-6(8)5-2-4(7)3-10-5/h4-5H,2-3,7H2,1H3/t4-,5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... |
US Patent US10800753 (2020)
BindingDB Entry DOI: 10.7270/Q2ZC85ZR |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50118886
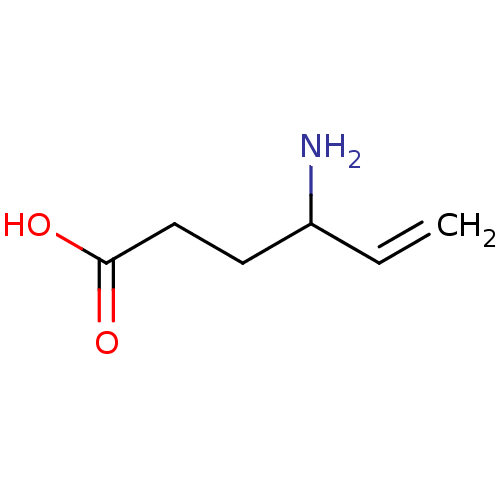
(4-Amino-hex-5-enoic acid | CHEMBL89598 | US1018980...)Show InChI InChI=1S/C6H11NO2/c1-2-5(7)3-4-6(8)9/h2,5H,1,3-4,7H2,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 8.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration dependent inactivation of Gamma-amino-N-butyrate transaminase |
J Med Chem 45: 4531-9 (2002)
BindingDB Entry DOI: 10.7270/Q2G1605T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM323800
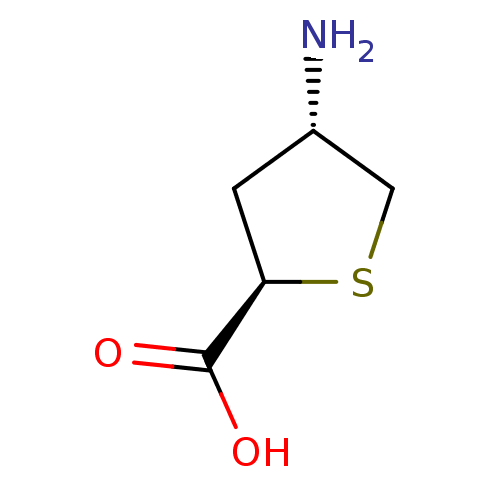
((2R,4S)-4-Aminotetrahydrothiophene-2-carboxylic ac...)Show InChI InChI=1S/C5H9NO2S/c6-3-1-4(5(7)8)9-2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.23E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... |
US Patent US10800753 (2020)
BindingDB Entry DOI: 10.7270/Q2ZC85ZR |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM323800

((2R,4S)-4-Aminotetrahydrothiophene-2-carboxylic ac...)Show InChI InChI=1S/C5H9NO2S/c6-3-1-4(5(7)8)9-2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.23E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... |
US Patent US10189807 (2019)
BindingDB Entry DOI: 10.7270/Q2C82CDG |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50118888
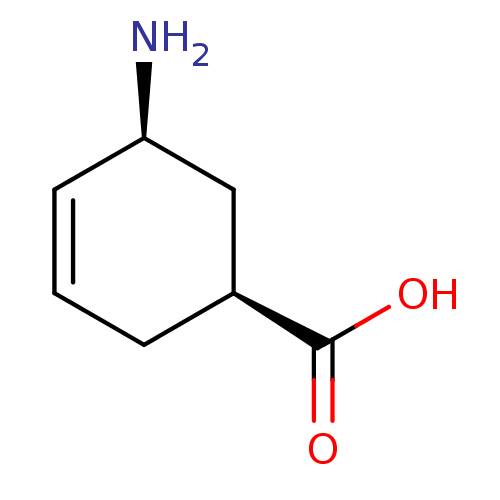
(5-Amino-cyclohex-3-enecarboxylic acid | CHEMBL3368...)Show InChI InChI=1S/C7H11NO2/c8-6-3-1-2-5(4-6)7(9)10/h1,3,5-6H,2,4,8H2,(H,9,10)/t5-,6+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration dependent inactivation of gGamma-amino-N-butyrate transaminase |
J Med Chem 45: 4531-9 (2002)
BindingDB Entry DOI: 10.7270/Q2G1605T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM323801
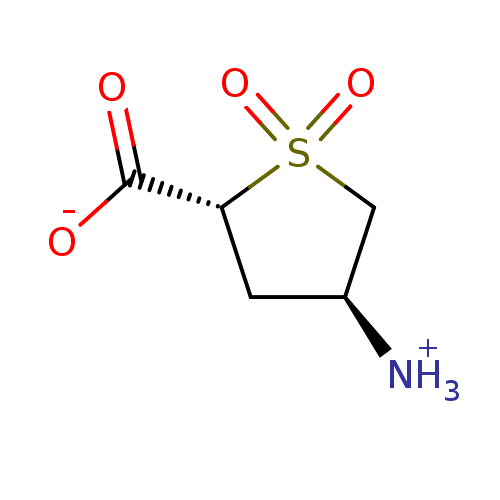
((4S)-4-Aminotetrahydrothiophene-2-carboxylic acid ...)Show InChI InChI=1S/C5H9NO4S/c6-3-1-4(5(7)8)11(9,10)2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... |
US Patent US10189807 (2019)
BindingDB Entry DOI: 10.7270/Q2C82CDG |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50357219
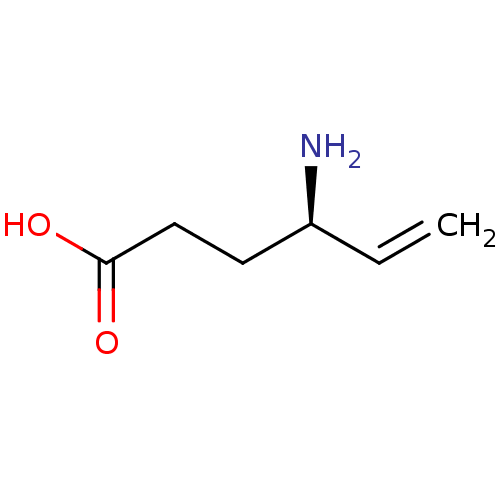
(CHEMBL1361446 | US10800753, Compound (S)-vigabatri...)Show InChI InChI=1S/C6H11NO2/c1-2-5(7)3-4-6(8)9/h2,5H,1,3-4,7H2,(H,8,9)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... |
US Patent US10800753 (2020)
BindingDB Entry DOI: 10.7270/Q2ZC85ZR |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50118886

(4-Amino-hex-5-enoic acid | CHEMBL89598 | US1018980...)Show InChI InChI=1S/C6H11NO2/c1-2-5(7)3-4-6(8)9/h2,5H,1,3-4,7H2,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inactivation of GABA-AT |
J Med Chem 55: 357-66 (2012)
Article DOI: 10.1021/jm201231w
BindingDB Entry DOI: 10.7270/Q2QF8V1F |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50118886

(4-Amino-hex-5-enoic acid | CHEMBL89598 | US1018980...)Show InChI InChI=1S/C6H11NO2/c1-2-5(7)3-4-6(8)9/h2,5H,1,3-4,7H2,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
US Patent
| 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... |
US Patent US10189807 (2019)
BindingDB Entry DOI: 10.7270/Q2C82CDG |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM466536
(US10800753, Compound 19)Show InChI InChI=1S/C5H9NO4S/c6-3-1-4(5(7)8)11(9,10)2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... |
US Patent US10800753 (2020)
BindingDB Entry DOI: 10.7270/Q2ZC85ZR |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM466538

(US10800753, Compound 21)Show InChI InChI=1S/C5H9NO2S/c6-3-1-4(5(7)8)9-2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... |
US Patent US10800753 (2020)
BindingDB Entry DOI: 10.7270/Q2ZC85ZR |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM323803
(US10189807, Compound 21)Show InChI InChI=1S/C5H9NO2S/c6-3-1-4(5(7)8)9-2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... |
US Patent US10189807 (2019)
BindingDB Entry DOI: 10.7270/Q2C82CDG |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM466537
(US10800753, Compound 20)Show InChI InChI=1S/C5H9NO2S/c6-3-1-4(5(7)8)9-2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... |
US Patent US10800753 (2020)
BindingDB Entry DOI: 10.7270/Q2ZC85ZR |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM323802
(US10189807, Compound 20)Show InChI InChI=1S/C5H9NO2S/c6-3-1-4(5(7)8)9-2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... |
US Patent US10189807 (2019)
BindingDB Entry DOI: 10.7270/Q2C82CDG |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50082127
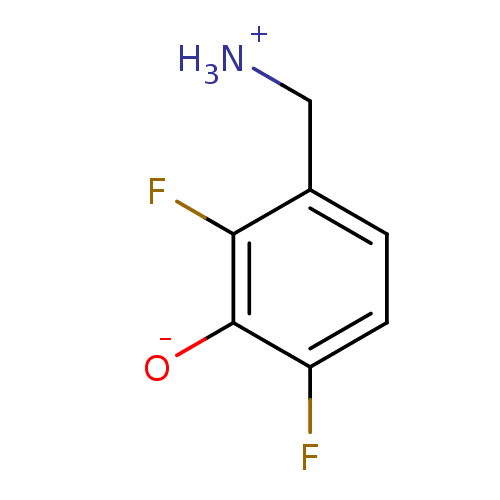
(3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...)Show InChI InChI=1S/C7H7F2NO/c8-5-2-1-4(3-10)6(9)7(5)11/h1-2,11H,3,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Competitive inhibition of GABA aminotransferase (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50082127

(3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...)Show InChI InChI=1S/C7H7F2NO/c8-5-2-1-4(3-10)6(9)7(5)11/h1-2,11H,3,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Competitive inhibition of GABA aminotransferase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM466539

(US10800753, Compound 22)Show InChI InChI=1S/C5H9NO4S/c6-3-1-4(5(7)8)11(9,10)2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 7.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0-50 mM concentrations of synthesized analogues using a couple... |
US Patent US10800753 (2020)
BindingDB Entry DOI: 10.7270/Q2ZC85ZR |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM323805
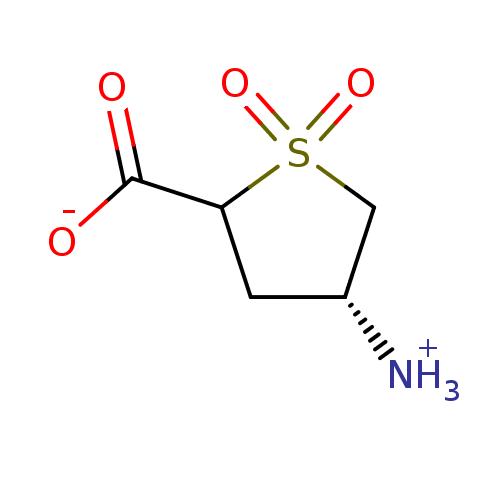
(US10189807, Compound 22)Show InChI InChI=1S/C5H9NO4S/c6-3-1-4(5(7)8)11(9,10)2-3/h3-4H,1-2,6H2,(H,7,8)/t3-,4?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 7.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confi... |
US Patent US10189807 (2019)
BindingDB Entry DOI: 10.7270/Q2C82CDG |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50073151
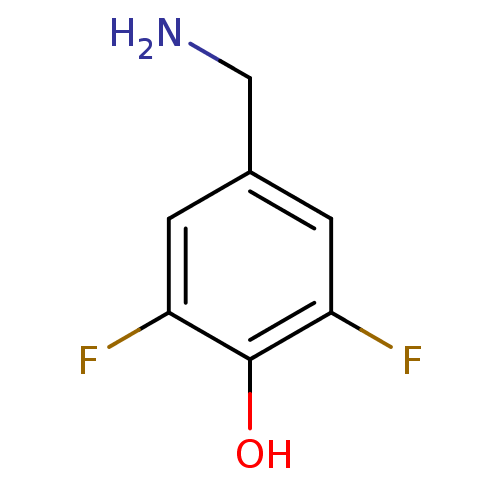
(4-(ammoniomethyl)-2,6-difluorobenzenolate | 4-Amin...)Show InChI InChI=1S/C7H7F2NO/c8-5-1-4(3-10)2-6(9)7(5)11/h1-2,11H,3,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Competitive inhibition of GABA aminotransferase |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50073151

(4-(ammoniomethyl)-2,6-difluorobenzenolate | 4-Amin...)Show InChI InChI=1S/C7H7F2NO/c8-5-1-4(3-10)2-6(9)7(5)11/h1-2,11H,3,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Competitive inhibition of GABA aminotransferase (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50118887
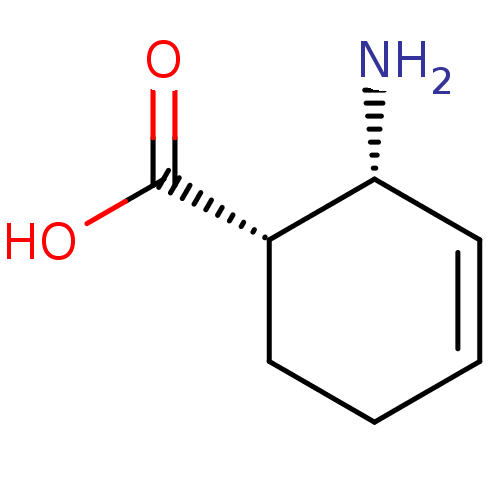
(2-Amino-cyclohex-3-enecarboxylic acid | CHEMBL1384...)Show InChI InChI=1S/C7H11NO2/c8-6-4-2-1-3-5(6)7(9)10/h2,4-6H,1,3,8H2,(H,9,10)/t5-,6+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration dependent inactivation of Gamma-amino-N-butyrate transaminase |
J Med Chem 45: 4531-9 (2002)
BindingDB Entry DOI: 10.7270/Q2G1605T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177407
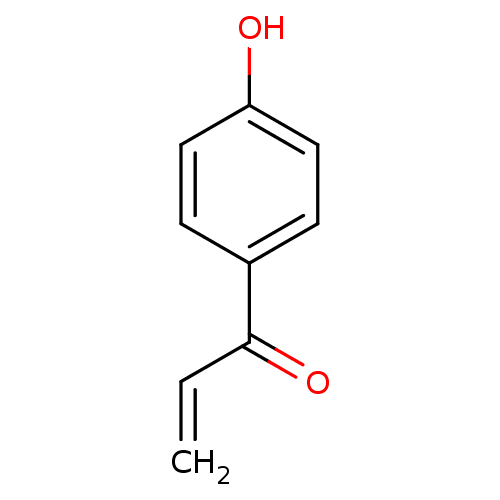
(1-(4-hydroxyphenyl)prop-2-en-1-one | 4-acryloylphe...)Show InChI InChI=1S/C9H8O2/c1-2-9(11)7-3-5-8(10)6-4-7/h2-6,10H,1H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177412
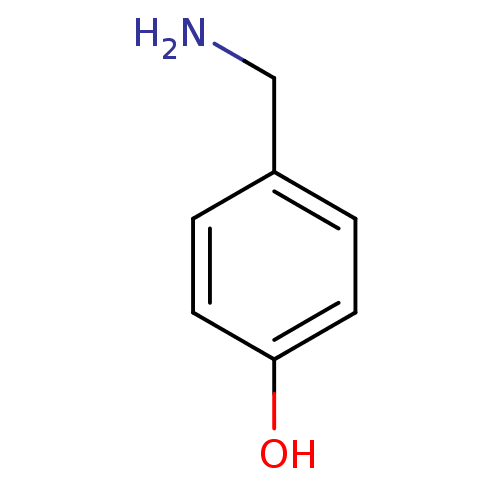
(4-(aminomethyl)phenol | 4-hydroxybenzylamine | CHE...)Show InChI InChI=1S/C7H9NO/c8-5-6-1-3-7(9)4-2-6/h1-4,9H,5,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177410

(4-hydroxy-3-nitrobenzaldehyde | CHEMBL378361)Show InChI InChI=1S/C7H5NO4/c9-4-5-1-2-7(10)6(3-5)8(11)12/h1-4,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177411
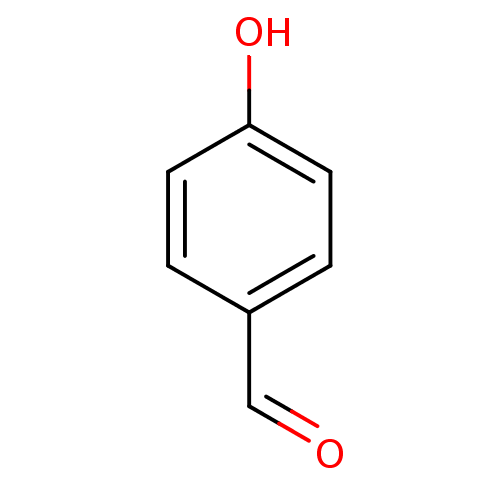
(4-formylphenol | 4-hydroxybenzaldehyde | CHEMBL141...)Show InChI InChI=1S/C7H6O2/c8-5-6-1-3-7(9)4-2-6/h1-5,9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177405
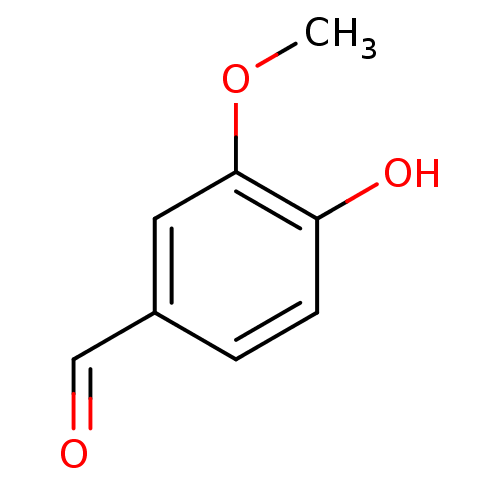
(3-methoxy-4-hydroxybenzaldehyde | 4-hydroxy-3-meth...)Show InChI InChI=1S/C8H8O3/c1-11-8-4-6(5-9)2-3-7(8)10/h2-5,10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177402
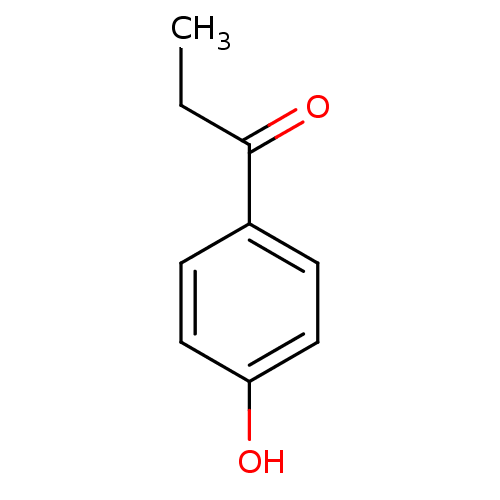
(1-(4-hydroxy-phenyl)-propan-1-one | 1-(4-hydroxyph...)Show InChI InChI=1S/C9H10O2/c1-2-9(11)7-3-5-8(10)6-4-7/h3-6,10H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177409
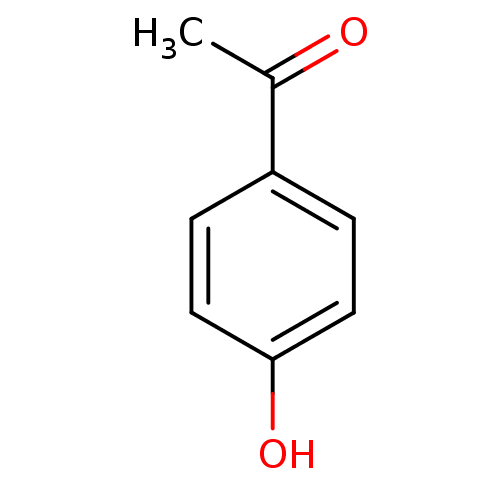
((4-hydroxyphenyl)ethan-1-one | 1-(4-hydroxyphenyl)...)Show InChI InChI=1S/C8H8O2/c1-6(9)7-2-4-8(10)5-3-7/h2-5,10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177404
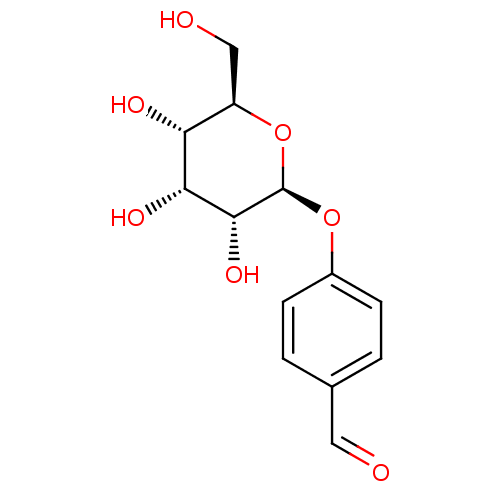
(4-((2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymet...)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(C=O)cc2)[C@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H16O7/c14-5-7-1-3-8(4-2-7)19-13-12(18)11(17)10(16)9(6-15)20-13/h1-5,9-13,15-18H,6H2/t9-,10-,11-,12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50145829
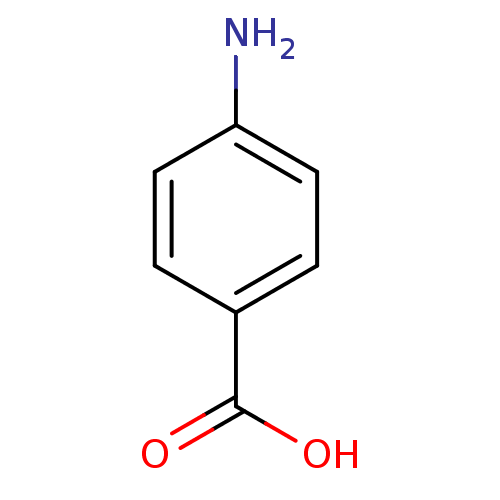
(4-Aminobenzoesaeure | 4-aminobenzoic acid | CHEMBL...)Show InChI InChI=1S/C7H7NO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,8H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177403
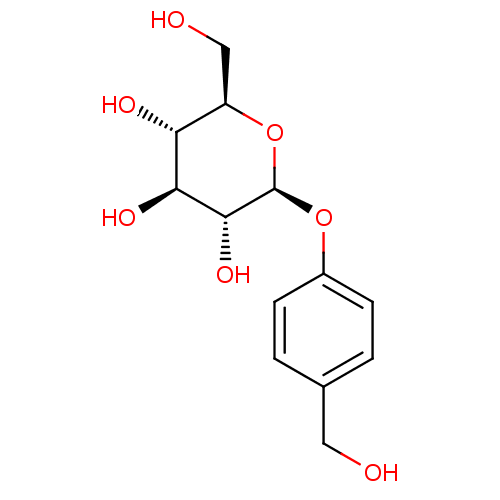
((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-(4-(hydroxyme...)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(CO)cc2)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C13H18O7/c14-5-7-1-3-8(4-2-7)19-13-12(18)11(17)10(16)9(6-15)20-13/h1-4,9-18H,5-6H2/t9-,10-,11+,12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177406
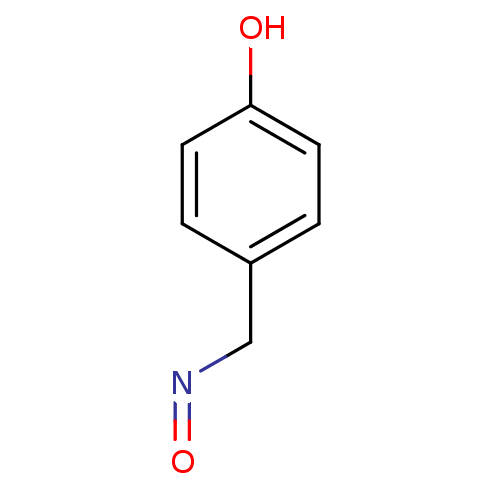
(4-hydroxybenzaldehyde oxime | CHEMBL202611)Show InChI InChI=1S/C7H7NO2/c9-7-3-1-6(2-4-7)5-8-10/h1-4,9H,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177408
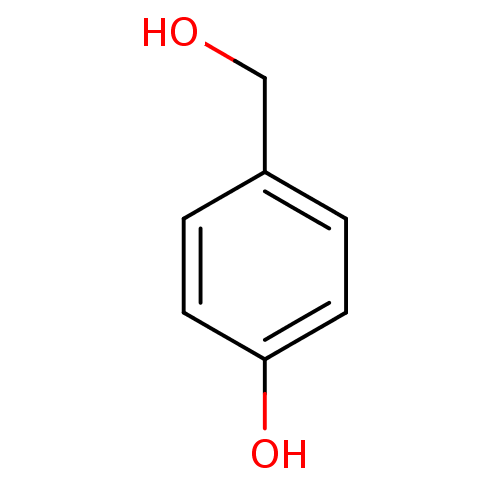
(4-(hydroxymethyl)phenol | 4-Hydroxybenzyl alcohol ...)Show InChI InChI=1S/C7H8O2/c8-5-6-1-3-7(9)4-2-6/h1-4,8-9H,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50139370
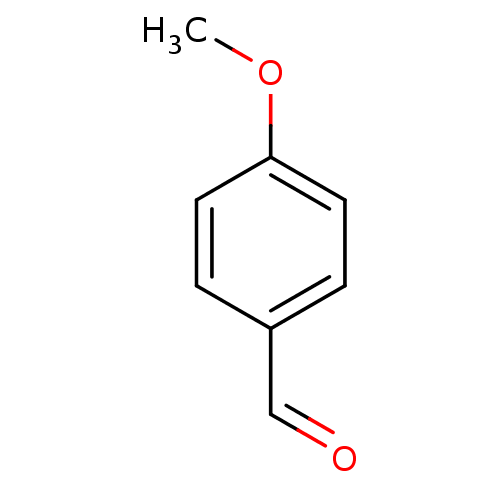
(4-Methoxy-benzaldehyde | 4-methoxybenzaldehyde | C...)Show InChI InChI=1S/C8H8O2/c1-10-8-4-2-7(6-9)3-5-8/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50177413
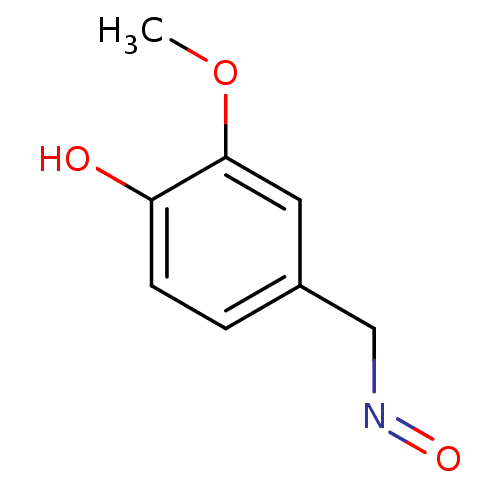
(4-hydroxy-3-methoxybenzaldehyde oxime | CHEMBL2024...)Show InChI InChI=1S/C8H9NO3/c1-12-8-4-6(5-9-11)2-3-7(8)10/h2-4,10H,5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM26194
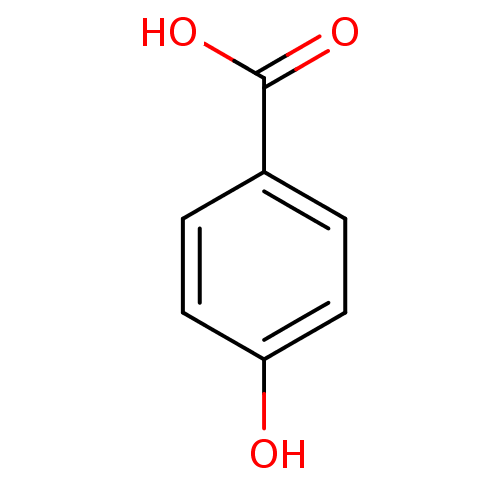
(4-Hydroxybenzoate, III | 4-hydroxybenzoic acid | C...)Show InChI InChI=1S/C7H6O3/c8-6-3-1-5(2-4-6)7(9)10/h1-4,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against GABAT |
Bioorg Med Chem Lett 16: 592-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.040
BindingDB Entry DOI: 10.7270/Q29G5MCH |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50376752
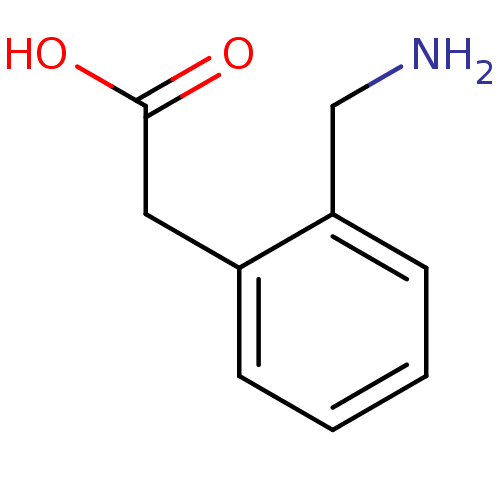
(CHEMBL535644)Show InChI InChI=1S/C9H11NO2/c10-6-8-4-2-1-3-7(8)5-9(11)12/h1-4H,5-6,10H2,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of GABA-AT |
Bioorg Med Chem Lett 18: 3122-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.060
BindingDB Entry DOI: 10.7270/Q22J6CRF |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50376753
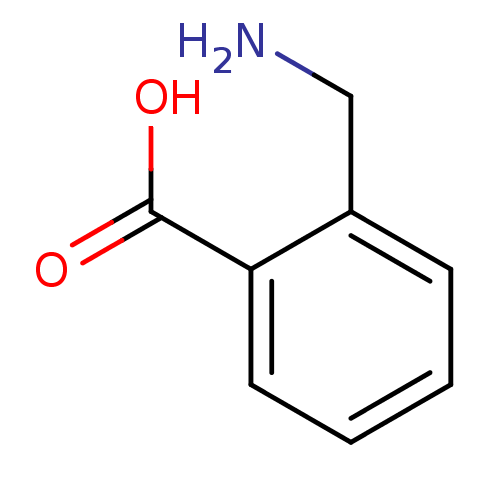
(CHEMBL535415)Show InChI InChI=1S/C8H9NO2/c9-5-6-3-1-2-4-7(6)8(10)11/h1-4H,5,9H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of GABA-AT |
Bioorg Med Chem Lett 18: 3122-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.060
BindingDB Entry DOI: 10.7270/Q22J6CRF |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50376754
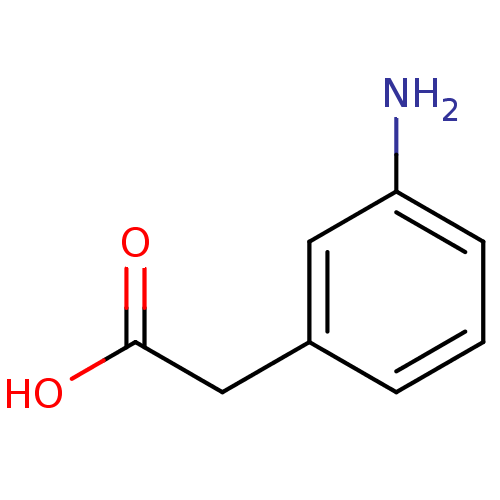
(CHEMBL535865)Show InChI InChI=1S/C8H9NO2/c9-7-3-1-2-6(4-7)5-8(10)11/h1-4H,5,9H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of GABA-AT |
Bioorg Med Chem Lett 18: 3122-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.060
BindingDB Entry DOI: 10.7270/Q22J6CRF |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50376750
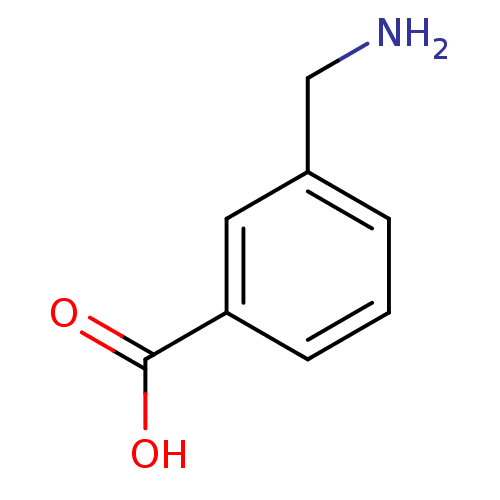
(CHEMBL541141)Show InChI InChI=1S/C8H9NO2/c9-5-6-2-1-3-7(4-6)8(10)11/h1-4H,5,9H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of GABA-AT |
Bioorg Med Chem Lett 18: 3122-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.060
BindingDB Entry DOI: 10.7270/Q22J6CRF |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50226518
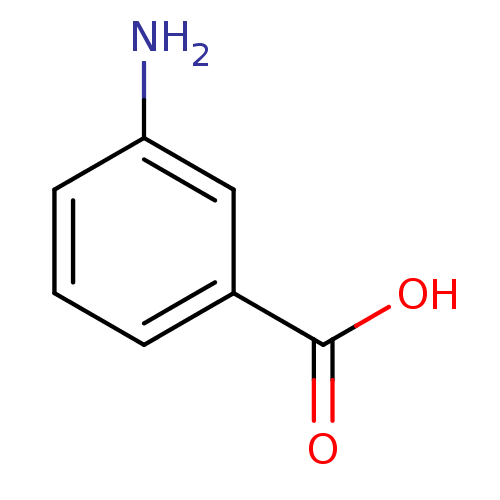
(3-Aminobenzoesaeure | 3-aminobenzoic acid | CHEMBL...)Show InChI InChI=1S/C7H7NO2/c8-6-3-1-2-5(4-6)7(9)10/h1-4H,8H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of GABA-AT |
Bioorg Med Chem Lett 18: 3122-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.060
BindingDB Entry DOI: 10.7270/Q22J6CRF |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50376751
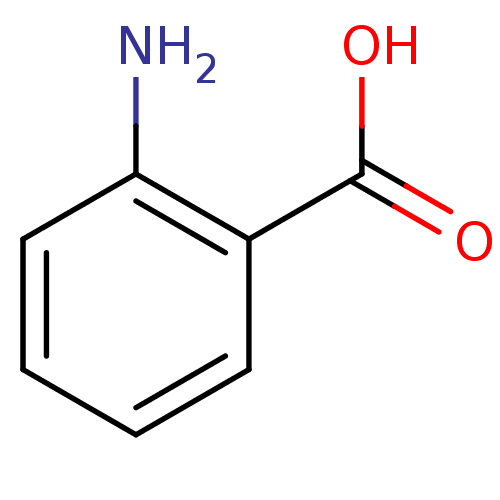
(ANTHRANILIC ACID)Show InChI InChI=1S/C7H7NO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,8H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of GABA-AT |
Bioorg Med Chem Lett 18: 3122-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.060
BindingDB Entry DOI: 10.7270/Q22J6CRF |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50376755
(CHEMBL535643)Show InChI InChI=1S/C9H11NO2/c10-6-8-3-1-2-7(4-8)5-9(11)12/h1-4H,5-6,10H2,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of GABA-AT |
Bioorg Med Chem Lett 18: 3122-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.060
BindingDB Entry DOI: 10.7270/Q22J6CRF |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50009001
(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.28E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of GABA transaminase (unknown origin) |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data