

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50009248 (2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-carboxylic acid amide::(2R,3S)-3-((4E,7E)-nona-4,7-dienoyl)oxirane-2-carboxamide::3-Nona-4,7-dienoyl-oxirane-2-carboxylic acid amide::3-Nona-4,7-dienoyl-oxirane-2-carboxylic acid amide(Cerulenin)::CERULENIN::CHEMBL45627
SMILES: C\C=C\C\C=C\CCC(=O)[C@H]1O[C@H]1C(N)=O
InChI Key: InChIKey=GVEZIHKRYBHEFX-NQQPLRFYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid synthase (Gallus gallus (Chicken)) | BDBM50009248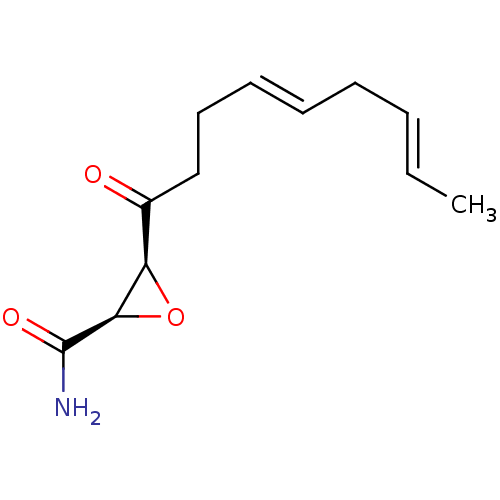 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Yeungnam University | Assay Description To each microtube (final volume: 100 μL), FAS was added (20-30 μg protein) in a buffer containing 100 mM potassium phosphate (pH 7.0), 2.5 ... | J Enzyme Inhib Med Chem 28: 565-8 (2013) Article DOI: 10.3109/14756366.2012.658786 BindingDB Entry DOI: 10.7270/Q2D21WHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII (Homo sapiens (Human)) | BDBM50009248 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... | Eur J Med Chem 98: 49-53 (2015) Article DOI: 10.1016/j.ejmech.2015.05.019 BindingDB Entry DOI: 10.7270/Q261121G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII (Homo sapiens (Human)) | BDBM50009248 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds Curated by ChEMBL | Assay Description Inhibition of F13-A in human plasma assessed as inhibition of fibrin clot formation by biotin incorporation assay in presence of 1 mM reduced GSH | Eur J Med Chem 98: 49-53 (2015) Article DOI: 10.1016/j.ejmech.2015.05.019 BindingDB Entry DOI: 10.7270/Q261121G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50009248 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of human FAS assessed as synthesis of long chain fatty acids from malonyl CoA after 60 mins by fluorescence assay | Bioorg Med Chem Lett 21: 5924-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.083 BindingDB Entry DOI: 10.7270/Q2MS3T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50009248 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Panlabs, Inc Curated by ChEMBL | Assay Description Inhibition of FAS in human ZR-75-1 cells by spectrophotometry | J Nat Prod 66: 1041-6 (2003) Article DOI: 10.1021/np030046g BindingDB Entry DOI: 10.7270/Q2GF0X86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human rhinovirus A protease (Human rhinovirus B) | BDBM50009248 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its affinity against HIV-2 protease (in vitro) | J Med Chem 34: 2305-14 (1991) BindingDB Entry DOI: 10.7270/Q2862H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50009248 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of FASN in human A375 cells | J Med Chem 54: 5615-38 (2011) Article DOI: 10.1021/jm2005805 BindingDB Entry DOI: 10.7270/Q21N8261 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50009248 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of FAS | Bioorg Med Chem Lett 16: 4738-42 (2006) Article DOI: 10.1016/j.bmcl.2006.07.018 BindingDB Entry DOI: 10.7270/Q2QV3NB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50009248 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi Curated by ChEMBL | Assay Description Inhibition of fatty acid synthase | J Nat Prod 66: 39-41 (2003) Article DOI: 10.1021/np020429z BindingDB Entry DOI: 10.7270/Q2DN47VH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50009248 ((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its affinity against HIV-1 protease in vitro | J Med Chem 34: 2305-14 (1991) BindingDB Entry DOI: 10.7270/Q2862H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||