

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 409.4 BDBM50174833 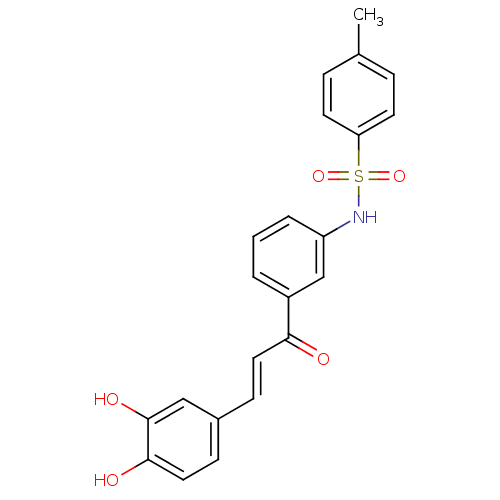 | Wt: 393.4 BDBM50174834 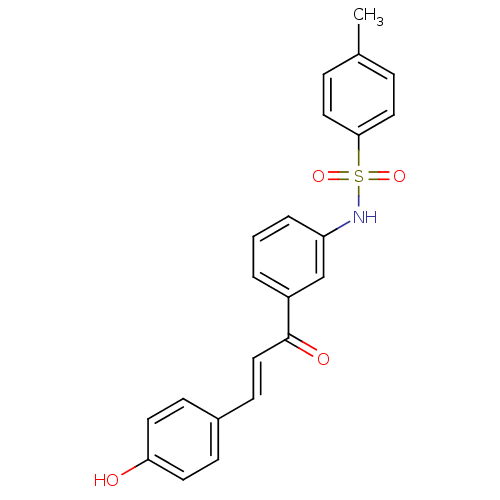 | Wt: 393.4 BDBM50174835 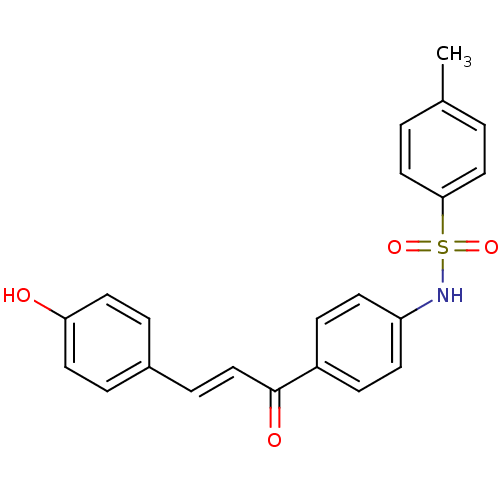 | Wt: 239.2 BDBM50174836 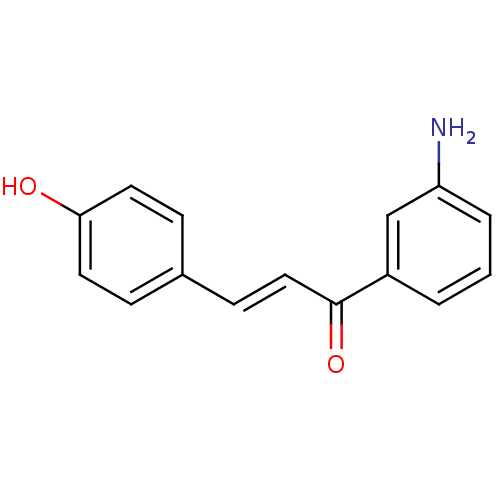 | Wt: 409.4 BDBM50174837 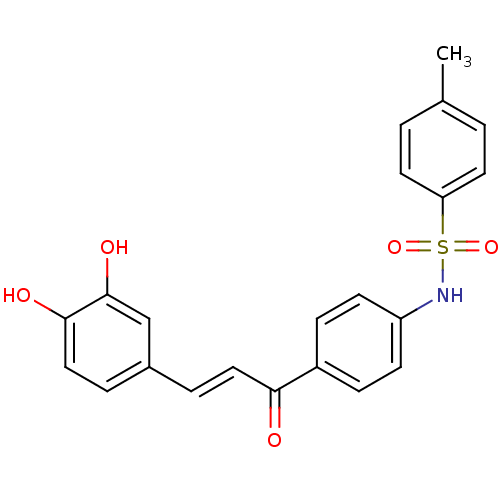 |
| Wt: 255.2 BDBM50174838 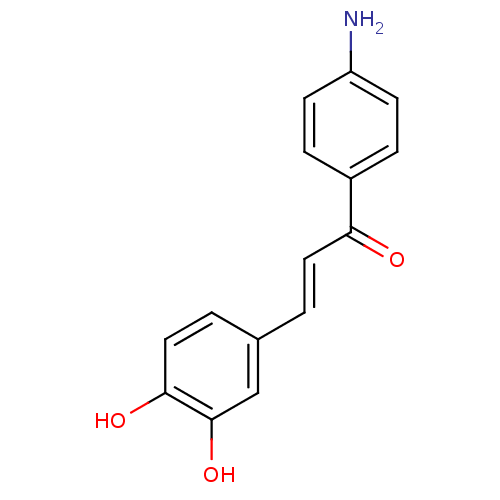 |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trans-sialidase (Trypanosoma cruzi) | BDBM50174833 (CHEMBL199442 | N-(3-(3-(3,4-dihydroxyphenyl)acrylo...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+3 | -32.0 | 2.50E+3 | n/a | n/a | n/a | n/a | 7.6 | 35 |
University of British Columbia | Assay Description Inhibition assay using trans-sialidase, a membrane-associated protein. | Chembiochem 10: 2475-9 (2009) Article DOI: 10.1002/cbic.200900108 BindingDB Entry DOI: 10.7270/Q2MG7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trans-sialidase (Trypanosoma cruzi) | BDBM50174837 (4'-(p-toluenesulfonamide)-3,4-dihydroxy chalcone |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60E+3 | -31.5 | 900 | n/a | n/a | n/a | n/a | 7.6 | 35 |
University of British Columbia | Assay Description Inhibition assay using trans-sialidase, a membrane-associated protein. | Chembiochem 10: 2475-9 (2009) Article DOI: 10.1002/cbic.200900108 BindingDB Entry DOI: 10.7270/Q2MG7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50174835 (4'-(4-toluenesulfonamido)-4-hydroxychalcone | 4'-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trans-sialidase (Trypanosoma cruzi) | BDBM50174837 (4'-(p-toluenesulfonamide)-3,4-dihydroxy chalcone |...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trans-sialidase using CF3MuSA as substrate by UV/visible spectrophotometric method | Eur J Med Chem 158: 25-33 (2018) Article DOI: 10.1016/j.ejmech.2018.08.089 BindingDB Entry DOI: 10.7270/Q2J105TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase (Bacillus licheniformis) | BDBM50174833 (CHEMBL199442 | N-(3-(3-(3,4-dihydroxyphenyl)acrylo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against Bacillus licheniformis alpha amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50174835 (4'-(4-toluenesulfonamido)-4-hydroxychalcone | 4'-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by spectrophotometric analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-amylase (Hordeum vulgare) | BDBM50174833 (CHEMBL199442 | N-(3-(3-(3,4-dihydroxyphenyl)acrylo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against barley beta amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trans-sialidase (Trypanosoma cruzi) | BDBM50174834 (CHEMBL382280 | N-(3-(3-(4-hydroxyphenyl)acryloyl)p...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | 7.6 | 35 |
University of British Columbia | Assay Description Inhibition assay using trans-sialidase, a membrane-associated protein. | Chembiochem 10: 2475-9 (2009) Article DOI: 10.1002/cbic.200900108 BindingDB Entry DOI: 10.7270/Q2MG7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase (Bacillus licheniformis) | BDBM50174834 (CHEMBL382280 | N-(3-(3-(4-hydroxyphenyl)acryloyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against Bacillus licheniformis alpha amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 10 (Rattus norvegicus) | BDBM50174835 (4'-(4-toluenesulfonamido)-4-hydroxychalcone | 4'-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of rat TREK2 channel -mediated current expressed in HEK293 at 60 mV holding potential by patch clamp method | Bioorg Med Chem Lett 20: 4237-9 (2010) Article DOI: 10.1016/j.bmcl.2010.05.033 BindingDB Entry DOI: 10.7270/Q25Q4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-amylase (Hordeum vulgare) | BDBM50174837 (4'-(p-toluenesulfonamide)-3,4-dihydroxy chalcone |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against barley beta amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trans-sialidase (Trypanosoma cruzi) | BDBM50174835 (4'-(4-toluenesulfonamido)-4-hydroxychalcone | 4'-(...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | 7.6 | 35 |
University of British Columbia | Assay Description Inhibition assay using trans-sialidase, a membrane-associated protein. | Chembiochem 10: 2475-9 (2009) Article DOI: 10.1002/cbic.200900108 BindingDB Entry DOI: 10.7270/Q2MG7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase (Bacillus licheniformis) | BDBM50174835 (4'-(4-toluenesulfonamido)-4-hydroxychalcone | 4'-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against Bacillus licheniformis alpha amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50174834 (CHEMBL382280 | N-(3-(3-(4-hydroxyphenyl)acryloyl)p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of bovine kidney alpha-fucosidase using PNPG as substrate incubated for 10 mins by spectrophotometric method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112034 BindingDB Entry DOI: 10.7270/Q2KW5KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50174833 (CHEMBL199442 | N-(3-(3-(3,4-dihydroxyphenyl)acrylo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of bovine kidney alpha-fucosidase using PNPG as substrate incubated for 10 mins by spectrophotometric method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112034 BindingDB Entry DOI: 10.7270/Q2KW5KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50174835 (4'-(4-toluenesulfonamido)-4-hydroxychalcone | 4'-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of bovine kidney alpha-fucosidase using PNPG as substrate incubated for 10 mins by spectrophotometric method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112034 BindingDB Entry DOI: 10.7270/Q2KW5KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50174837 (4'-(p-toluenesulfonamide)-3,4-dihydroxy chalcone |...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of bovine kidney alpha-fucosidase using PNPG as substrate incubated for 10 mins by spectrophotometric method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112034 BindingDB Entry DOI: 10.7270/Q2KW5KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50174837 (4'-(p-toluenesulfonamide)-3,4-dihydroxy chalcone |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of jack bean alpha-mannosidase using PNPG as substrate incubated for 10 mins by spectrophotometric method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112034 BindingDB Entry DOI: 10.7270/Q2KW5KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50174835 (4'-(4-toluenesulfonamido)-4-hydroxychalcone | 4'-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of jack bean alpha-mannosidase using PNPG as substrate incubated for 10 mins by spectrophotometric method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112034 BindingDB Entry DOI: 10.7270/Q2KW5KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50174833 (CHEMBL199442 | N-(3-(3-(3,4-dihydroxyphenyl)acrylo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of jack bean alpha-mannosidase using PNPG as substrate incubated for 10 mins by spectrophotometric method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112034 BindingDB Entry DOI: 10.7270/Q2KW5KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50174834 (CHEMBL382280 | N-(3-(3-(4-hydroxyphenyl)acryloyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of jack bean alpha-mannosidase using PNPG as substrate incubated for 10 mins by spectrophotometric method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112034 BindingDB Entry DOI: 10.7270/Q2KW5KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-amylase (Hordeum vulgare) | BDBM50174838 (1-(4-aminophenyl)-3-(3,4-dihydroxyphenyl)prop-2-en...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against barley beta amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase (Bacillus licheniformis) | BDBM50174837 (4'-(p-toluenesulfonamide)-3,4-dihydroxy chalcone |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against Bacillus licheniformis alpha amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-amylase (Hordeum vulgare) | BDBM50174834 (CHEMBL382280 | N-(3-(3-(4-hydroxyphenyl)acryloyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against barley beta amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-amylase (Hordeum vulgare) | BDBM50174836 (1-(3-aminophenyl)-3-(4-hydroxyphenyl)prop-2-en-1-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against barley beta amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-amylase (Hordeum vulgare) | BDBM50174835 (4'-(4-toluenesulfonamido)-4-hydroxychalcone | 4'-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibitory activity against barley beta amylase | Bioorg Med Chem Lett 15: 5514-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.087 BindingDB Entry DOI: 10.7270/Q2FX7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||