 Found 103 hits in this display
Found 103 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete
Curated by ChEMBL
| Assay Description
Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin |
Bioorg Med Chem 19: 2842-9 (2011)
Article DOI: 10.1016/j.bmc.2011.03.042
BindingDB Entry DOI: 10.7270/Q2V69JXK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human 15-lipoxygenase |
J Med Chem 54: 5485-97 (2011)
Article DOI: 10.1021/jm2005089
BindingDB Entry DOI: 10.7270/Q25T3KVT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin |
Bioorg Med Chem 19: 2842-9 (2011)
Article DOI: 10.1016/j.bmc.2011.03.042
BindingDB Entry DOI: 10.7270/Q2V69JXK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human platelet-type 12-lipoxygenase |
J Med Chem 54: 5485-97 (2011)
Article DOI: 10.1021/jm2005089
BindingDB Entry DOI: 10.7270/Q25T3KVT |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of JMJD2E relative to alpha-ketoglutarate |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Rattus norvegicus) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma |
J Med Chem 34: 1503-5 (1991)
BindingDB Entry DOI: 10.7270/Q22Z14G6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against 15-lipoxygenase in rat polymorphonuclear leukocytes |
J Med Chem 34: 1503-5 (1991)
BindingDB Entry DOI: 10.7270/Q22Z14G6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01140
BindingDB Entry DOI: 10.7270/Q2445RJD |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
South China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 integrase strand transfer activity using 32P 5' end-labeled linear 21'mer as substrate preincubated for 30 mins prior... |
Bioorg Med Chem 22: 3146-58 (2014)
Article DOI: 10.1016/j.bmc.2014.04.016
BindingDB Entry DOI: 10.7270/Q2XG9SPQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Universidad de Santiago
| Assay Description
The reactions were done in a volume of 2 mL and constantly stirred using a magnetic stir bar at room temperature (23 °C). Reactions with the crude, a... |
Chem Biol Drug Des 86: 114-21 (2015)
Article DOI: 10.1111/cbdd.12469
BindingDB Entry DOI: 10.7270/Q2PZ57JF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Chile
Curated by ChEMBL
| Assay Description
Inhibition of 12-hLO |
Bioorg Med Chem 15: 7408-25 (2007)
Article DOI: 10.1016/j.bmc.2007.07.036
BindingDB Entry DOI: 10.7270/Q2RN37KN |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
This is a review article. Please point to the original journal. |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00409
BindingDB Entry DOI: 10.7270/Q2J1069F |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01140
BindingDB Entry DOI: 10.7270/Q2445RJD |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115860
BindingDB Entry DOI: 10.7270/Q2154N01 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115860
BindingDB Entry DOI: 10.7270/Q2154N01 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115860
BindingDB Entry DOI: 10.7270/Q2154N01 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114508
BindingDB Entry DOI: 10.7270/Q2PR8119 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM375076
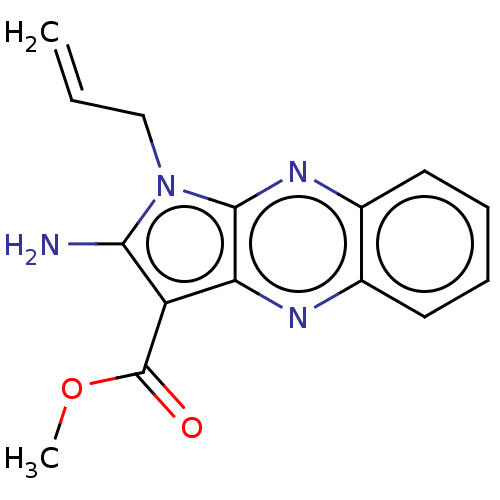
(US10252984, Table 2.37)Show InChI InChI=1S/C15H14N4O2/c1-3-8-19-13(16)11(15(20)21-2)12-14(19)18-10-7-5-4-6-9(10)17-12/h3-7H,1,8,16H2,2H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 973 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
TBD |
J Pharmacol Exp Ther 314: 1310-21 (2005)
BindingDB Entry DOI: 10.7270/Q2BV7JXQ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01140
BindingDB Entry DOI: 10.7270/Q2445RJD |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
IC50 was measured as concentration required to inhibit 50% of HIV-integrase cleavage |
J Med Chem 38: 890-7 (1995)
BindingDB Entry DOI: 10.7270/Q2959J6V |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM375084
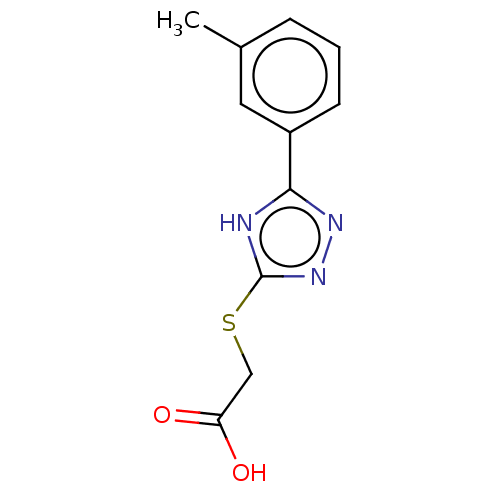
(US10252984, Table 2.45)Show InChI InChI=1S/C11H11N3O2S/c1-7-3-2-4-8(5-7)10-12-11(14-13-10)17-6-9(15)16/h2-5H,6H2,1H3,(H,15,16)(H,12,13,14) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
TBD |
J Pharmacol Exp Ther 314: 1310-21 (2005)
BindingDB Entry DOI: 10.7270/Q2BV7JXQ |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM375077

(US10252984, Table 2.38)Show InChI InChI=1S/C15H15N5/c1-2-3-8-20-14(17)10(9-16)13-15(20)19-12-7-5-4-6-11(12)18-13/h4-7H,2-3,8,17H2,1H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
TBD |
J Pharmacol Exp Ther 314: 1310-21 (2005)
BindingDB Entry DOI: 10.7270/Q2BV7JXQ |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Strasbourg
| Assay Description
H-DXR was pre-incubated during 2 min in the presence of the inhibitors at different concentrations and DXP (480 µM). NADPH (160 µM final concentratio... |
Bioorg Chem 59: 140-4 (2015)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q2XS5T4J |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM375063
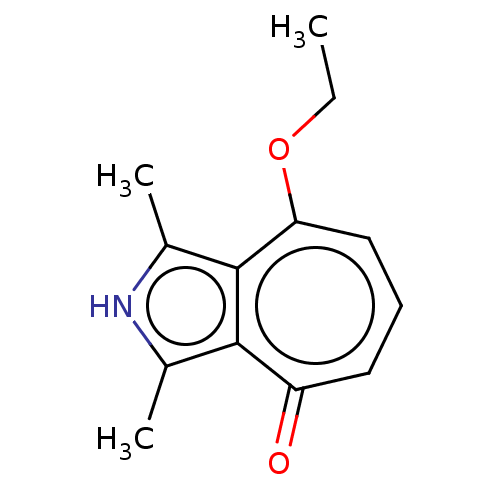
(US10252984, Table 2.24)Show InChI InChI=1S/C13H15NO2/c1-4-16-11-7-5-6-10(15)12-8(2)14-9(3)13(11)12/h5-7,14H,4H2,1-3H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
TBD |
J Pharmacol Exp Ther 314: 1310-21 (2005)
BindingDB Entry DOI: 10.7270/Q2BV7JXQ |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
South China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged HIV-1 integrase assessed as decrease in integrase-Flag-LEDGF/p75 interaction preincubated with enzyme for 30 mins followed ... |
Bioorg Med Chem 22: 3146-58 (2014)
Article DOI: 10.1016/j.bmc.2014.04.016
BindingDB Entry DOI: 10.7270/Q2XG9SPQ |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase assessed as decrease in uric acid production by spectrophotometry |
J Nat Prod 61: 71-6 (1998)
Article DOI: 10.1021/np970237h
BindingDB Entry DOI: 10.7270/Q29C6Z93 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of JMJD2E |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Universidad de Santiago de Chile
| Assay Description
All reactions were carried in a volume of 2 mL stirred at 23°C with approximately 2.040 Units of 12-hLOX, 4.200 Units of 15-hLOX-1, and 6.600 Un... |
Chem Biol Drug Des 82: 317-25 (2013)
Article DOI: 10.1111/cbdd.12157
BindingDB Entry DOI: 10.7270/Q2PZ57F3 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of cow milk xanthine oxidase |
J Nat Prod 51: 345-348 (1988)
Article DOI: 10.1021/np50056a030
BindingDB Entry DOI: 10.7270/Q2DJ5FNN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of YES (unknown origin) |
Eur J Med Chem 166: 186-196 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.043
BindingDB Entry DOI: 10.7270/Q20C50BS |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ehime University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-induced elevation in PAI1 production in HUVEC by ELISA |
J Nat Prod 60: 598-601 (1997)
Article DOI: 10.1021/np970035l
BindingDB Entry DOI: 10.7270/Q20C4ZK4 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
| Assay Description
The kinase assay was performed using the EMD Millipore KinaseProfiler service assay protocol. Aurora B kinase was supplied by EMD Millipore Corp. The... |
Chem Biol Drug Des 85: 574-85 (2015)
Article DOI: 10.1111/cbdd.12445
BindingDB Entry DOI: 10.7270/Q2M61J08 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
IC50 was measured as concentration required to inhibit 50% of HIV-integrase integration |
J Med Chem 38: 890-7 (1995)
BindingDB Entry DOI: 10.7270/Q2959J6V |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS method |
Drug Metab Dispos 39: 322-9 (2011)
Article DOI: 10.1124/dmd.110.035030
BindingDB Entry DOI: 10.7270/Q2PC343R |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM375086
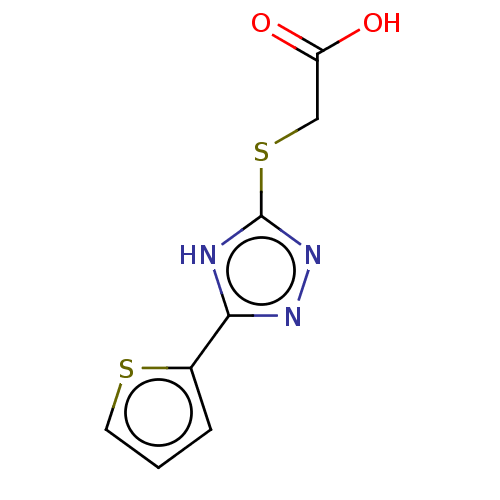
(US10252984, Table 2.47)Show InChI InChI=1S/C8H7N3O2S2/c12-6(13)4-15-8-9-7(10-11-8)5-2-1-3-14-5/h1-3H,4H2,(H,12,13)(H,9,10,11) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
TBD |
J Pharmacol Exp Ther 314: 1310-21 (2005)
BindingDB Entry DOI: 10.7270/Q2BV7JXQ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00805
BindingDB Entry DOI: 10.7270/Q2PC36DM |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 5.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Cdk1/cyclin B |
Bioorg Med Chem 16: 7128-33 (2008)
Article DOI: 10.1016/j.bmc.2008.06.055
BindingDB Entry DOI: 10.7270/Q2JQ11XQ |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS method |
Drug Metab Dispos 39: 322-9 (2011)
Article DOI: 10.1124/dmd.110.035030
BindingDB Entry DOI: 10.7270/Q2PC343R |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM375075
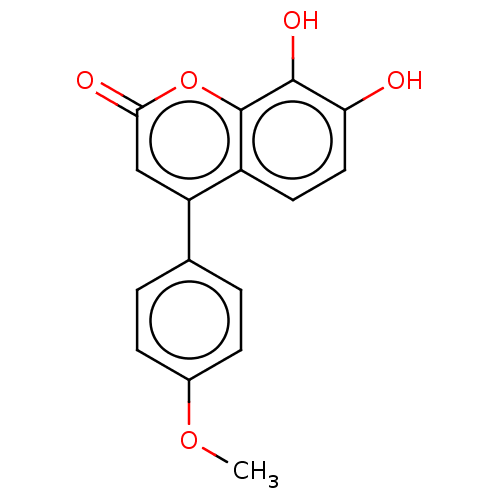
(US10252984, Table 2.36)Show InChI InChI=1S/C16H12O5/c1-20-10-4-2-9(3-5-10)12-8-14(18)21-16-11(12)6-7-13(17)15(16)19/h2-8,17,19H,1H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
TBD |
J Pharmacol Exp Ther 314: 1310-21 (2005)
BindingDB Entry DOI: 10.7270/Q2BV7JXQ |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
South China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 integrase 3'-processing activity using 32P 5' end-labeled linear 21'mer as substrate preincubated for 30 mins prior t... |
Bioorg Med Chem 22: 3146-58 (2014)
Article DOI: 10.1016/j.bmc.2014.04.016
BindingDB Entry DOI: 10.7270/Q2XG9SPQ |
More data for this
Ligand-Target Pair | |
Alpha-synuclein
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human alpha-synuclein filament formation expressed in Escherichia coli BL21(DE3) cells incubated for 72 hrs by thioflavin S based fluor... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.01.045
BindingDB Entry DOI: 10.7270/Q2H998V9 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
TBD |
J Pharmacol Exp Ther 314: 1310-21 (2005)
BindingDB Entry DOI: 10.7270/Q2BV7JXQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00614
BindingDB Entry DOI: 10.7270/Q2Z89HD2 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Chile
Curated by ChEMBL
| Assay Description
Inhibition of 15-hLO1 |
Bioorg Med Chem 15: 7408-25 (2007)
Article DOI: 10.1016/j.bmc.2007.07.036
BindingDB Entry DOI: 10.7270/Q2RN37KN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Tripura University, Suryamaninagar 799022, Tripura, India. Electronic address: dindabtu@gmail.com.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in baculovirus infected insect cells using 7-benzyloxy-4-trifluoromethylcoumarin as substrate measured after 45 ... |
Eur J Med Chem 131: 68-80 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.004
BindingDB Entry DOI: 10.7270/Q2R49T6V |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase
 Purchase
Purchase Purchase
Purchase