 Found 148 hits with Last Name = 'aumelas' and Initial = 'a'
Found 148 hits with Last Name = 'aumelas' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089316
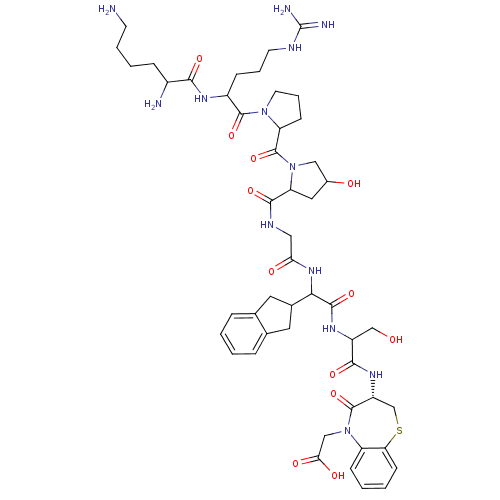
(CHEMBL410068 | H-Lys-Arg-Pro-Hyp-Gly-Igl-Ser-D-BT-...)Show SMILES NCCCCC(N)C(=O)NC(CCCNC(N)=N)C(=O)N1CCCC1C(=O)N1CC(O)CC1C(=O)NCC(=O)NC(C1Cc2ccccc2C1)C(=O)NC(CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C49H69N13O12S/c50-16-6-5-11-31(51)42(68)56-32(12-7-17-54-49(52)53)46(72)60-18-8-14-36(60)48(74)61-23-30(64)21-37(61)44(70)55-22-39(65)59-41(29-19-27-9-1-2-10-28(27)20-29)45(71)57-33(25-63)43(69)58-34-26-75-38-15-4-3-13-35(38)62(47(34)73)24-40(66)67/h1-4,9-10,13,15,29-34,36-37,41,63-64H,5-8,11-12,14,16-26,50-51H2,(H,55,70)(H,56,68)(H,57,71)(H,58,69)(H,59,65)(H,66,67)(H4,52,53,54)/t30?,31?,32?,33?,34-,36?,37?,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human cloned B1 receptor was determined using [3H]-[des-Arg10-Leu9]-kallidin as radioligand |
J Med Chem 43: 2387-94 (2000)
BindingDB Entry DOI: 10.7270/Q2N87913 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038601
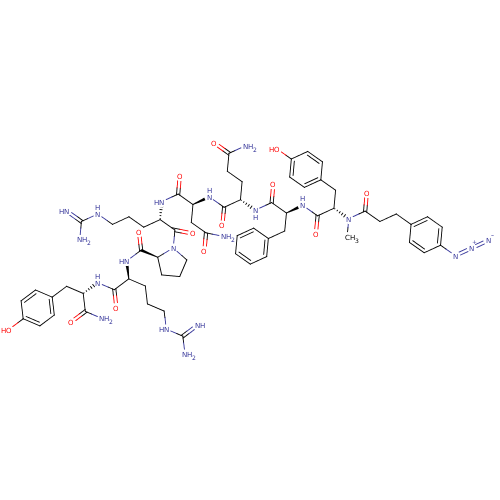
(4-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)28-19-36-13-20-40(21-14-36)80-81-71)50(34-39-17-24-42(85)25-18-39)60(95)79-47(33-37-8-3-2-4-9-37)57(92)74-44(26-27-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(11-6-30-73-63(69)70)61(96)83-31-7-12-49(83)59(94)75-43(10-5-29-72-62(67)68)55(90)77-46(54(66)89)32-38-15-22-41(84)23-16-38/h2-4,8-9,13-18,20-25,43-50,84-85H,5-7,10-12,19,26-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant determined from vasopressin induced inositol phosphates accumulation performed on WRK1 cell line of V1a receptor subty... |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038602
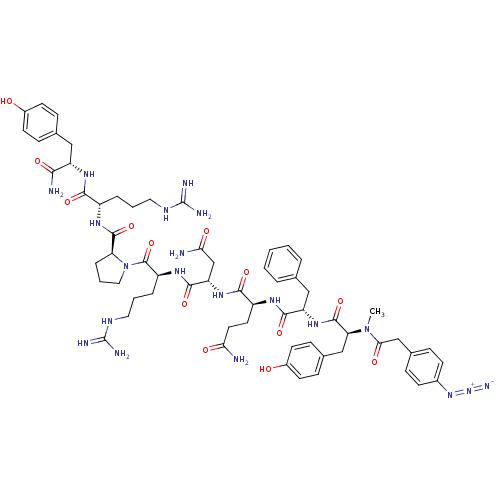
(4-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-13-19-39(20-14-38)79-80-70)49(32-37-17-23-41(84)24-18-37)59(94)78-46(31-35-8-3-2-4-9-35)56(91)73-43(25-26-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(11-6-28-72-62(68)69)60(95)82-29-7-12-48(82)58(93)74-42(10-5-27-71-61(66)67)54(89)76-45(53(65)88)30-36-15-21-40(83)22-16-36/h2-4,8-9,13-24,42-49,83-84H,5-7,10-12,25-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Inhibition constant for V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038603
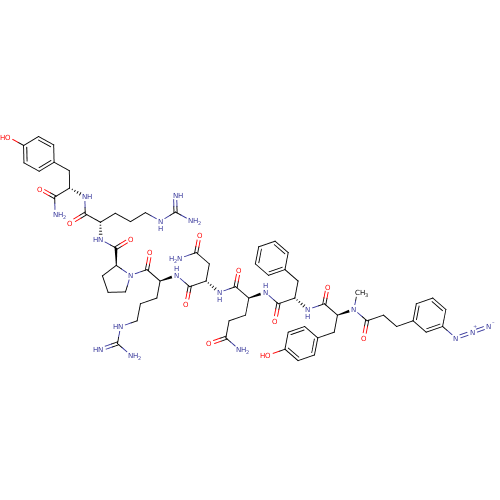
(3-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)27-20-37-11-5-12-40(31-37)80-81-71)50(34-39-18-23-42(85)24-19-39)60(95)79-47(33-36-9-3-2-4-10-36)57(92)74-44(25-26-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(14-7-29-73-63(69)70)61(96)83-30-8-15-49(83)59(94)75-43(13-6-28-72-62(67)68)55(90)77-46(54(66)89)32-38-16-21-41(84)22-17-38/h2-5,9-12,16-19,21-24,31,43-50,84-85H,6-8,13-15,20,25-30,32-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant determined from vasopressin induced inositol phosphates accumulation performed on WRK1 cell line of V1a receptor subty... |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038603

(3-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)27-20-37-11-5-12-40(31-37)80-81-71)50(34-39-18-23-42(85)24-19-39)60(95)79-47(33-36-9-3-2-4-10-36)57(92)74-44(25-26-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(14-7-29-73-63(69)70)61(96)83-30-8-15-49(83)59(94)75-43(13-6-28-72-62(67)68)55(90)77-46(54(66)89)32-38-16-21-41(84)22-17-38/h2-5,9-12,16-19,21-24,31,43-50,84-85H,6-8,13-15,20,25-30,32-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Inhibition constant for V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038599
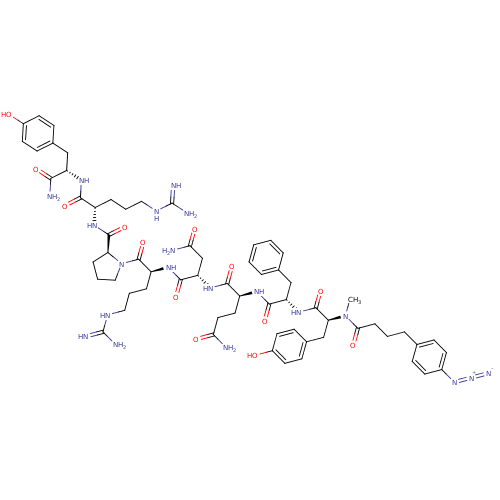
(4-N3-C6H4CH2CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C64H86N20O13/c1-83(54(89)15-5-11-37-16-22-41(23-17-37)81-82-72)51(35-40-20-26-43(86)27-21-40)61(96)80-48(34-38-9-3-2-4-10-38)58(93)75-45(28-29-52(65)87)57(92)79-49(36-53(66)88)59(94)77-46(13-7-31-74-64(70)71)62(97)84-32-8-14-50(84)60(95)76-44(12-6-30-73-63(68)69)56(91)78-47(55(67)90)33-39-18-24-42(85)25-19-39/h2-4,9-10,16-27,44-51,85-86H,5-8,11-15,28-36H2,1H3,(H2,65,87)(H2,66,88)(H2,67,90)(H,75,93)(H,76,95)(H,77,94)(H,78,91)(H,79,92)(H,80,96)(H4,68,69,73)(H4,70,71,74)/t44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Inhibition constant for V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038602

(4-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-13-19-39(20-14-38)79-80-70)49(32-37-17-23-41(84)24-18-37)59(94)78-46(31-35-8-3-2-4-9-35)56(91)73-43(25-26-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(11-6-28-72-62(68)69)60(95)82-29-7-12-48(82)58(93)74-42(10-5-27-71-61(66)67)54(89)76-45(53(65)88)30-36-15-21-40(83)22-16-36/h2-4,8-9,13-24,42-49,83-84H,5-7,10-12,25-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant determined from vasopressin induced inositol phosphates accumulation performed on WRK1 cell line of V1a receptor subty... |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038604
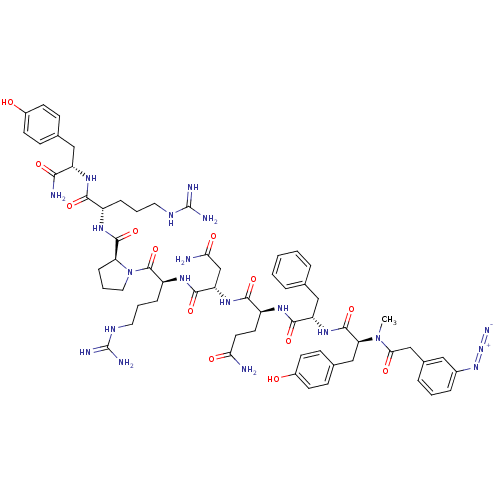
(3-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-11-5-12-39(29-38)79-80-70)49(32-37-18-22-41(84)23-19-37)59(94)78-46(31-35-9-3-2-4-10-35)56(91)73-43(24-25-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(14-7-27-72-62(68)69)60(95)82-28-8-15-48(82)58(93)74-42(13-6-26-71-61(66)67)54(89)76-45(53(65)88)30-36-16-20-40(83)21-17-36/h2-5,9-12,16-23,29,42-49,83-84H,6-8,13-15,24-28,30-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant determined from vasopressin induced inositol phosphates accumulation performed on WRK1 cell line of V1a receptor subty... |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50103475

(Pmp-Tyr-Ile-Thr-Asn-Cys-Pro-Orn-phe(I,N3)-NH2 | Pm...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)CC1(CCCCC1)SCCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N1CCCC1C(=O)NC(CCCN)C(=O)NC(Cc1ccc(N=[N+]=[N-])c(I)c1)C(N)=O Show InChI InChI=1S/C57H84IN15O13S2/c1-5-31(2)47(69-52(81)40(26-33-13-16-35(86-4)17-14-33)64-46(77)29-57(20-7-6-8-21-57)88-24-19-44(60)75)54(83)70-48(32(3)74)55(84)67-41(28-45(61)76)51(80)68-42(30-87)56(85)73-23-10-12-43(73)53(82)65-38(11-9-22-59)50(79)66-39(49(62)78)27-34-15-18-37(71-72-63)36(58)25-34/h13-18,25,31-32,38-43,47-48,74,87H,5-12,19-24,26-30,59H2,1-4H3,(H2,60,75)(H2,61,76)(H2,62,78)(H,64,77)(H,65,82)(H,66,79)(H,67,84)(H,68,80)(H,69,81)(H,70,83)/t31-,32+,38?,39?,40-,41-,42-,43?,47-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity towards OT receptor in CHO cells expressing the human OT receptor |
J Med Chem 44: 3022-30 (2001)
BindingDB Entry DOI: 10.7270/Q29S1Q9F |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038604

(3-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-11-5-12-39(29-38)79-80-70)49(32-37-18-22-41(84)23-19-37)59(94)78-46(31-35-9-3-2-4-10-35)56(91)73-43(24-25-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(14-7-27-72-62(68)69)60(95)82-28-8-15-48(82)58(93)74-42(13-6-26-71-61(66)67)54(89)76-45(53(65)88)30-36-16-20-40(83)21-17-36/h2-5,9-12,16-23,29,42-49,83-84H,6-8,13-15,24-28,30-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Inhibition constant for V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038604

(3-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-11-5-12-39(29-38)79-80-70)49(32-37-18-22-41(84)23-19-37)59(94)78-46(31-35-9-3-2-4-10-35)56(91)73-43(24-25-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(14-7-27-72-62(68)69)60(95)82-28-8-15-48(82)58(93)74-42(13-6-26-71-61(66)67)54(89)76-45(53(65)88)30-36-16-20-40(83)21-17-36/h2-5,9-12,16-23,29,42-49,83-84H,6-8,13-15,24-28,30-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Inhibition constant for V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038599

(4-N3-C6H4CH2CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C64H86N20O13/c1-83(54(89)15-5-11-37-16-22-41(23-17-37)81-82-72)51(35-40-20-26-43(86)27-21-40)61(96)80-48(34-38-9-3-2-4-10-38)58(93)75-45(28-29-52(65)87)57(92)79-49(36-53(66)88)59(94)77-46(13-7-31-74-64(70)71)62(97)84-32-8-14-50(84)60(95)76-44(12-6-30-73-63(68)69)56(91)78-47(55(67)90)33-39-18-24-42(85)25-19-39/h2-4,9-10,16-27,44-51,85-86H,5-8,11-15,28-36H2,1H3,(H2,65,87)(H2,66,88)(H2,67,90)(H,75,93)(H,76,95)(H,77,94)(H,78,91)(H,79,92)(H,80,96)(H4,68,69,73)(H4,70,71,74)/t44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant determined from vasopressin induced inositol phosphates accumulation performed on WRK1 cell line of V1a receptor subty... |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038601

(4-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)28-19-36-13-20-40(21-14-36)80-81-71)50(34-39-17-24-42(85)25-18-39)60(95)79-47(33-37-8-3-2-4-9-37)57(92)74-44(26-27-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(11-6-30-73-63(69)70)61(96)83-31-7-12-49(83)59(94)75-43(10-5-29-72-62(67)68)55(90)77-46(54(66)89)32-38-15-22-41(84)23-16-38/h2-4,8-9,13-18,20-25,43-50,84-85H,5-7,10-12,19,26-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Inhibition constant for V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50103477
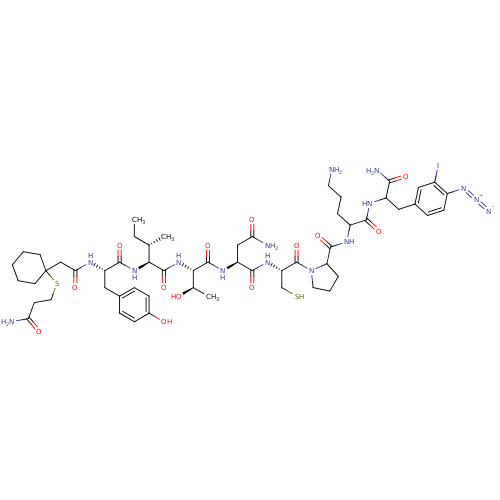
(CHEMBL386180 | Pmp-Tyr-Ile-Thr-Asn-Cys-Pro-Orn-phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CC1(CCCCC1)SCCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N1CCCC1C(=O)NC(CCCN)C(=O)NC(Cc1ccc(N=[N+]=[N-])c(I)c1)C(N)=O Show InChI InChI=1S/C56H82IN15O13S2/c1-4-30(2)46(68-51(81)39(25-32-12-15-34(74)16-13-32)63-45(77)28-56(19-6-5-7-20-56)87-23-18-43(59)75)53(83)69-47(31(3)73)54(84)66-40(27-44(60)76)50(80)67-41(29-86)55(85)72-22-9-11-42(72)52(82)64-37(10-8-21-58)49(79)65-38(48(61)78)26-33-14-17-36(70-71-62)35(57)24-33/h12-17,24,30-31,37-42,46-47,73-74,86H,4-11,18-23,25-29,58H2,1-3H3,(H2,59,75)(H2,60,76)(H2,61,78)(H,63,77)(H,64,82)(H,65,79)(H,66,84)(H,67,80)(H,68,81)(H,69,83)/t30-,31+,37?,38?,39-,40-,41-,42?,46-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity towards OT receptor in CHO cells expressing the human OT receptor |
J Med Chem 44: 3022-30 (2001)
BindingDB Entry DOI: 10.7270/Q29S1Q9F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50038604

(3-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-11-5-12-39(29-38)79-80-70)49(32-37-18-22-41(84)23-19-37)59(94)78-46(31-35-9-3-2-4-10-35)56(91)73-43(24-25-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(14-7-27-72-62(68)69)60(95)82-28-8-15-48(82)58(93)74-42(13-6-26-71-61(66)67)54(89)76-45(53(65)88)30-36-16-20-40(83)21-17-36/h2-5,9-12,16-23,29,42-49,83-84H,6-8,13-15,24-28,30-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at OT receptor of rat mamary glands |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50038603

(3-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)27-20-37-11-5-12-40(31-37)80-81-71)50(34-39-18-23-42(85)24-19-39)60(95)79-47(33-36-9-3-2-4-10-36)57(92)74-44(25-26-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(14-7-29-73-63(69)70)61(96)83-30-8-15-49(83)59(94)75-43(13-6-28-72-62(67)68)55(90)77-46(54(66)89)32-38-16-21-41(84)22-17-38/h2-5,9-12,16-19,21-24,31,43-50,84-85H,6-8,13-15,20,25-30,32-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at OT receptor of rat mamary glands |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50038601

(4-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)28-19-36-13-20-40(21-14-36)80-81-71)50(34-39-17-24-42(85)25-18-39)60(95)79-47(33-37-8-3-2-4-9-37)57(92)74-44(26-27-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(11-6-30-73-63(69)70)61(96)83-31-7-12-49(83)59(94)75-43(10-5-29-72-62(67)68)55(90)77-46(54(66)89)32-38-15-22-41(84)23-16-38/h2-4,8-9,13-18,20-25,43-50,84-85H,5-7,10-12,19,26-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at V2 receptor of rat kidney membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50103478

(CHEMBL406670 | Pmp-Tyr(Me)-Ile-Thr-Asn-Cys-Pro-Orn...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)CC1(CCCCC1)SCCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)NCC(=O)NC(CCCN)C(=O)NC(Cc1ccc(N=[N+]=[N-])c(I)c1)C(N)=O Show InChI InChI=1S/C54H80IN15O13S2/c1-5-29(2)45(67-51(80)38(23-31-11-14-33(83-4)15-12-31)63-43(74)26-54(18-7-6-8-19-54)85-21-17-41(57)72)52(81)68-46(30(3)71)53(82)65-39(25-42(58)73)50(79)66-40(28-84)48(77)61-27-44(75)62-36(10-9-20-56)49(78)64-37(47(59)76)24-32-13-16-35(69-70-60)34(55)22-32/h11-16,22,29-30,36-40,45-46,71,84H,5-10,17-21,23-28,56H2,1-4H3,(H2,57,72)(H2,58,73)(H2,59,76)(H,61,77)(H,62,75)(H,63,74)(H,64,78)(H,65,82)(H,66,79)(H,67,80)(H,68,81)/t29-,30+,36?,37?,38-,39-,40-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity towards OT receptor in CHO cells expressing the human OT receptor |
J Med Chem 44: 3022-30 (2001)
BindingDB Entry DOI: 10.7270/Q29S1Q9F |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50038599

(4-N3-C6H4CH2CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C64H86N20O13/c1-83(54(89)15-5-11-37-16-22-41(23-17-37)81-82-72)51(35-40-20-26-43(86)27-21-40)61(96)80-48(34-38-9-3-2-4-10-38)58(93)75-45(28-29-52(65)87)57(92)79-49(36-53(66)88)59(94)77-46(13-7-31-74-64(70)71)62(97)84-32-8-14-50(84)60(95)76-44(12-6-30-73-63(68)69)56(91)78-47(55(67)90)33-39-18-24-42(85)25-19-39/h2-4,9-10,16-27,44-51,85-86H,5-8,11-15,28-36H2,1H3,(H2,65,87)(H2,66,88)(H2,67,90)(H,75,93)(H,76,95)(H,77,94)(H,78,91)(H,79,92)(H,80,96)(H4,68,69,73)(H4,70,71,74)/t44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at V2 receptor of rat kidney membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50038602

(4-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-13-19-39(20-14-38)79-80-70)49(32-37-17-23-41(84)24-18-37)59(94)78-46(31-35-8-3-2-4-9-35)56(91)73-43(25-26-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(11-6-28-72-62(68)69)60(95)82-29-7-12-48(82)58(93)74-42(10-5-27-71-61(66)67)54(89)76-45(53(65)88)30-36-15-21-40(83)22-16-36/h2-4,8-9,13-24,42-49,83-84H,5-7,10-12,25-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at OT receptor of rat mamary glands |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50038602

(4-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-13-19-39(20-14-38)79-80-70)49(32-37-17-23-41(84)24-18-37)59(94)78-46(31-35-8-3-2-4-9-35)56(91)73-43(25-26-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(11-6-28-72-62(68)69)60(95)82-29-7-12-48(82)58(93)74-42(10-5-27-71-61(66)67)54(89)76-45(53(65)88)30-36-15-21-40(83)22-16-36/h2-4,8-9,13-24,42-49,83-84H,5-7,10-12,25-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at V2 receptor of rat kidney membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089316

(CHEMBL410068 | H-Lys-Arg-Pro-Hyp-Gly-Igl-Ser-D-BT-...)Show SMILES NCCCCC(N)C(=O)NC(CCCNC(N)=N)C(=O)N1CCCC1C(=O)N1CC(O)CC1C(=O)NCC(=O)NC(C1Cc2ccccc2C1)C(=O)NC(CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C49H69N13O12S/c50-16-6-5-11-31(51)42(68)56-32(12-7-17-54-49(52)53)46(72)60-18-8-14-36(60)48(74)61-23-30(64)21-37(61)44(70)55-22-39(65)59-41(29-19-27-9-1-2-10-28(27)20-29)45(71)57-33(25-63)43(69)58-34-26-75-38-15-4-3-13-35(38)62(47(34)73)24-40(66)67/h1-4,9-10,13,15,29-34,36-37,41,63-64H,5-8,11-12,14,16-26,50-51H2,(H,55,70)(H,56,68)(H,57,71)(H,58,69)(H,59,65)(H,66,67)(H4,52,53,54)/t30?,31?,32?,33?,34-,36?,37?,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human cloned B2 receptor was determined using [3H]-BK as radioligand |
J Med Chem 43: 2387-94 (2000)
BindingDB Entry DOI: 10.7270/Q2N87913 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50038604

(3-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-11-5-12-39(29-38)79-80-70)49(32-37-18-22-41(84)23-19-37)59(94)78-46(31-35-9-3-2-4-10-35)56(91)73-43(24-25-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(14-7-27-72-62(68)69)60(95)82-28-8-15-48(82)58(93)74-42(13-6-26-71-61(66)67)54(89)76-45(53(65)88)30-36-16-20-40(83)21-17-36/h2-5,9-12,16-23,29,42-49,83-84H,6-8,13-15,24-28,30-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at V2 receptor of rat kidney membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038605
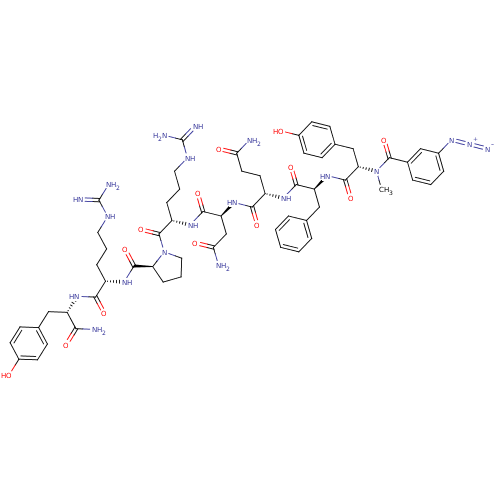
(3-N3-C6H4CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)c1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C61H80N20O13/c1-80(58(93)37-11-5-12-38(32-37)78-79-69)48(31-36-18-22-40(83)23-19-36)57(92)77-45(30-34-9-3-2-4-10-34)54(89)72-42(24-25-49(62)84)53(88)76-46(33-50(63)85)55(90)74-43(14-7-27-71-61(67)68)59(94)81-28-8-15-47(81)56(91)73-41(13-6-26-70-60(65)66)52(87)75-44(51(64)86)29-35-16-20-39(82)21-17-35/h2-5,9-12,16-23,32,41-48,82-83H,6-8,13-15,24-31,33H2,1H3,(H2,62,84)(H2,63,85)(H2,64,86)(H,72,89)(H,73,91)(H,74,90)(H,75,87)(H,76,88)(H,77,92)(H4,65,66,70)(H4,67,68,71)/t41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Inhibition constant for V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50103476
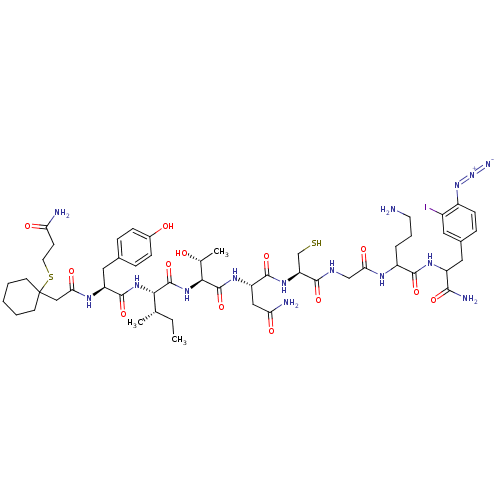
(CHEMBL412559 | Pmp-Tyr-Ile-Thr-Asn-Cys-Pro-Orn-phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CC1(CCCCC1)SCCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)NCC(=O)NC(CCCN)C(=O)NC(Cc1ccc(N=[N+]=[N-])c(I)c1)C(N)=O Show InChI InChI=1S/C53H78IN15O13S2/c1-4-28(2)44(66-50(80)37(22-30-10-13-32(71)14-11-30)62-42(74)25-53(17-6-5-7-18-53)84-20-16-40(56)72)51(81)67-45(29(3)70)52(82)64-38(24-41(57)73)49(79)65-39(27-83)47(77)60-26-43(75)61-35(9-8-19-55)48(78)63-36(46(58)76)23-31-12-15-34(68-69-59)33(54)21-31/h10-15,21,28-29,35-39,44-45,70-71,83H,4-9,16-20,22-27,55H2,1-3H3,(H2,56,72)(H2,57,73)(H2,58,76)(H,60,77)(H,61,75)(H,62,74)(H,63,78)(H,64,82)(H,65,79)(H,66,80)(H,67,81)/t28-,29+,35?,36?,37-,38-,39-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity towards OT receptor in CHO cells expressing the human OT receptor |
J Med Chem 44: 3022-30 (2001)
BindingDB Entry DOI: 10.7270/Q29S1Q9F |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50038599

(4-N3-C6H4CH2CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C64H86N20O13/c1-83(54(89)15-5-11-37-16-22-41(23-17-37)81-82-72)51(35-40-20-26-43(86)27-21-40)61(96)80-48(34-38-9-3-2-4-10-38)58(93)75-45(28-29-52(65)87)57(92)79-49(36-53(66)88)59(94)77-46(13-7-31-74-64(70)71)62(97)84-32-8-14-50(84)60(95)76-44(12-6-30-73-63(68)69)56(91)78-47(55(67)90)33-39-18-24-42(85)25-19-39/h2-4,9-10,16-27,44-51,85-86H,5-8,11-15,28-36H2,1H3,(H2,65,87)(H2,66,88)(H2,67,90)(H,75,93)(H,76,95)(H,77,94)(H,78,91)(H,79,92)(H,80,96)(H4,68,69,73)(H4,70,71,74)/t44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at OT receptor of rat mamary glands |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038600
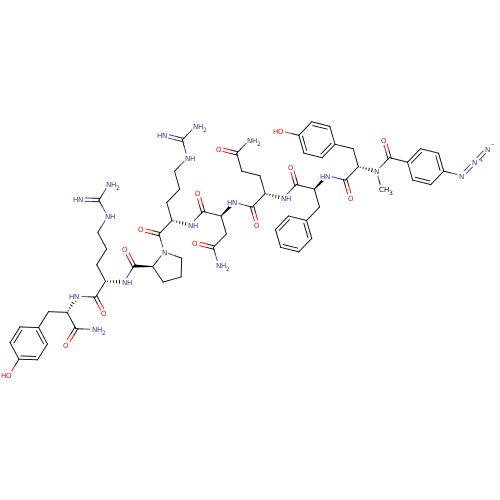
(4-N3-C6H4CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)c1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C61H80N20O13/c1-80(58(93)37-17-19-38(20-18-37)78-79-69)48(32-36-15-23-40(83)24-16-36)57(92)77-45(31-34-8-3-2-4-9-34)54(89)72-42(25-26-49(62)84)53(88)76-46(33-50(63)85)55(90)74-43(11-6-28-71-61(67)68)59(94)81-29-7-12-47(81)56(91)73-41(10-5-27-70-60(65)66)52(87)75-44(51(64)86)30-35-13-21-39(82)22-14-35/h2-4,8-9,13-24,41-48,82-83H,5-7,10-12,25-33H2,1H3,(H2,62,84)(H2,63,85)(H2,64,86)(H,72,89)(H,73,91)(H,74,90)(H,75,87)(H,76,88)(H,77,92)(H4,65,66,70)(H4,67,68,71)/t41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Inhibition constant for V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089315
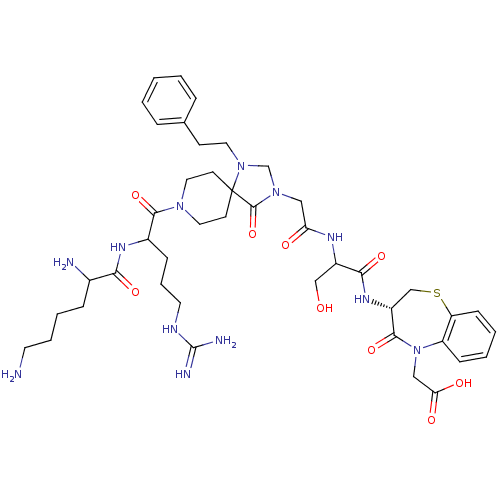
(CHEMBL80461 | {3-[2-(2-{8-[2-(2,6-Diamino-hexanoyl...)Show SMILES NCCCCC(N)C(=O)NC(CCCNC(N)=N)C(=O)N1CCC2(CC1)N(CCc1ccccc1)CN(CC(=O)NC(CO)C(=O)N[C@@H]1CSc3ccccc3N(CC(O)=O)C1=O)C2=O Show InChI InChI=1S/C43H62N12O9S/c44-18-7-6-11-29(45)37(60)50-30(12-8-19-48-42(46)47)39(62)52-21-16-43(17-22-52)41(64)53(27-54(43)20-15-28-9-2-1-3-10-28)23-35(57)49-31(25-56)38(61)51-32-26-65-34-14-5-4-13-33(34)55(40(32)63)24-36(58)59/h1-5,9-10,13-14,29-32,56H,6-8,11-12,15-27,44-45H2,(H,49,57)(H,50,60)(H,51,61)(H,58,59)(H4,46,47,48)/t29?,30?,31?,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 24.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human cloned B1 receptor was determined using [3H]-[des-Arg10-Leu9]-kallidin as radioligand |
J Med Chem 43: 2387-94 (2000)
BindingDB Entry DOI: 10.7270/Q2N87913 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50047222
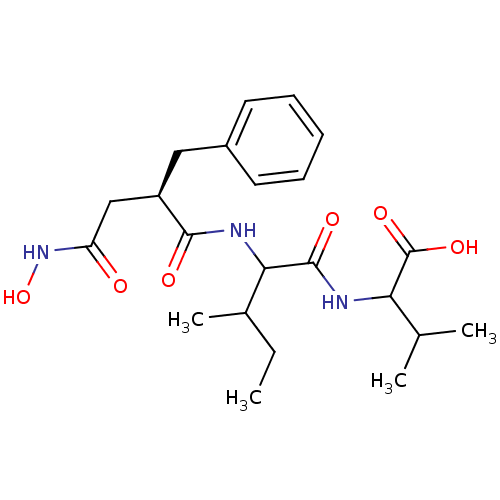
(2-[2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-...)Show SMILES CCC(C)C(NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(=O)NC(C(C)C)C(O)=O Show InChI InChI=1S/C22H33N3O6/c1-5-14(4)19(21(28)23-18(13(2)3)22(29)30)24-20(27)16(12-17(26)25-31)11-15-9-7-6-8-10-15/h6-10,13-14,16,18-19,31H,5,11-12H2,1-4H3,(H,23,28)(H,24,27)(H,25,26)(H,29,30)/t14?,16-,18?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CCIPE-Faculté de Pharmacie
Curated by ChEMBL
| Assay Description
Inhibitory activity against endopeptidase 24.15 |
J Med Chem 36: 1369-79 (1993)
BindingDB Entry DOI: 10.7270/Q2736PZX |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50038601

(4-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)28-19-36-13-20-40(21-14-36)80-81-71)50(34-39-17-24-42(85)25-18-39)60(95)79-47(33-37-8-3-2-4-9-37)57(92)74-44(26-27-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(11-6-30-73-63(69)70)61(96)83-31-7-12-49(83)59(94)75-43(10-5-29-72-62(67)68)55(90)77-46(54(66)89)32-38-15-22-41(84)23-16-38/h2-4,8-9,13-18,20-25,43-50,84-85H,5-7,10-12,19,26-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 37.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at OT receptor of rat mamary glands |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50047222

(2-[2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-...)Show SMILES CCC(C)C(NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(=O)NC(C(C)C)C(O)=O Show InChI InChI=1S/C22H33N3O6/c1-5-14(4)19(21(28)23-18(13(2)3)22(29)30)24-20(27)16(12-17(26)25-31)11-15-9-7-6-8-10-15/h6-10,13-14,16,18-19,31H,5,11-12H2,1-4H3,(H,23,28)(H,24,27)(H,25,26)(H,29,30)/t14?,16-,18?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CCIPE-Faculté de Pharmacie
Curated by ChEMBL
| Assay Description
Inhibitory activity against endopeptidase 24.11 |
J Med Chem 36: 1369-79 (1993)
BindingDB Entry DOI: 10.7270/Q2736PZX |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50047222

(2-[2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-...)Show SMILES CCC(C)C(NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(=O)NC(C(C)C)C(O)=O Show InChI InChI=1S/C22H33N3O6/c1-5-14(4)19(21(28)23-18(13(2)3)22(29)30)24-20(27)16(12-17(26)25-31)11-15-9-7-6-8-10-15/h6-10,13-14,16,18-19,31H,5,11-12H2,1-4H3,(H,23,28)(H,24,27)(H,25,26)(H,29,30)/t14?,16-,18?,19?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CCIPE-Faculté de Pharmacie
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leucine aminopeptidase |
J Med Chem 36: 1369-79 (1993)
BindingDB Entry DOI: 10.7270/Q2736PZX |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50047222

(2-[2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-...)Show SMILES CCC(C)C(NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(=O)NC(C(C)C)C(O)=O Show InChI InChI=1S/C22H33N3O6/c1-5-14(4)19(21(28)23-18(13(2)3)22(29)30)24-20(27)16(12-17(26)25-31)11-15-9-7-6-8-10-15/h6-10,13-14,16,18-19,31H,5,11-12H2,1-4H3,(H,23,28)(H,24,27)(H,25,26)(H,29,30)/t14?,16-,18?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CCIPE-Faculté de Pharmacie
Curated by ChEMBL
| Assay Description
Inhibitory activity against endopeptidase 24.16 |
J Med Chem 36: 1369-79 (1993)
BindingDB Entry DOI: 10.7270/Q2736PZX |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50047223
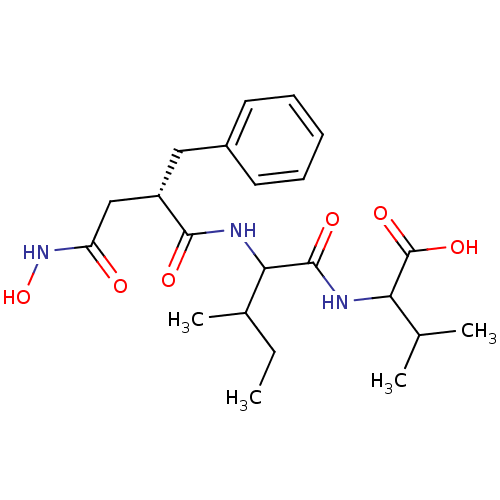
(2-[2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-...)Show SMILES CCC(C)C(NC(=O)[C@H](CC(=O)NO)Cc1ccccc1)C(=O)NC(C(C)C)C(O)=O Show InChI InChI=1S/C22H33N3O6/c1-5-14(4)19(21(28)23-18(13(2)3)22(29)30)24-20(27)16(12-17(26)25-31)11-15-9-7-6-8-10-15/h6-10,13-14,16,18-19,31H,5,11-12H2,1-4H3,(H,23,28)(H,24,27)(H,25,26)(H,29,30)/t14?,16-,18?,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CCIPE-Faculté de Pharmacie
Curated by ChEMBL
| Assay Description
Inhibitory activity against endopeptidase 24.16 |
J Med Chem 36: 1369-79 (1993)
BindingDB Entry DOI: 10.7270/Q2736PZX |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089315

(CHEMBL80461 | {3-[2-(2-{8-[2-(2,6-Diamino-hexanoyl...)Show SMILES NCCCCC(N)C(=O)NC(CCCNC(N)=N)C(=O)N1CCC2(CC1)N(CCc1ccccc1)CN(CC(=O)NC(CO)C(=O)N[C@@H]1CSc3ccccc3N(CC(O)=O)C1=O)C2=O Show InChI InChI=1S/C43H62N12O9S/c44-18-7-6-11-29(45)37(60)50-30(12-8-19-48-42(46)47)39(62)52-21-16-43(17-22-52)41(64)53(27-54(43)20-15-28-9-2-1-3-10-28)23-35(57)49-31(25-56)38(61)51-32-26-65-34-14-5-4-13-33(34)55(40(32)63)24-36(58)59/h1-5,9-10,13-14,29-32,56H,6-8,11-12,15-27,44-45H2,(H,49,57)(H,50,60)(H,51,61)(H,58,59)(H4,46,47,48)/t29?,30?,31?,32-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human cloned B2 receptor was determined using [3H]-BK as radioligand |
J Med Chem 43: 2387-94 (2000)
BindingDB Entry DOI: 10.7270/Q2N87913 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50038603

(3-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)27-20-37-11-5-12-40(31-37)80-81-71)50(34-39-18-23-42(85)24-19-39)60(95)79-47(33-36-9-3-2-4-10-36)57(92)74-44(25-26-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(14-7-29-73-63(69)70)61(96)83-30-8-15-49(83)59(94)75-43(13-6-28-72-62(67)68)55(90)77-46(54(66)89)32-38-16-21-41(84)22-17-38/h2-5,9-12,16-19,21-24,31,43-50,84-85H,6-8,13-15,20,25-30,32-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for inhibition constant at V2 receptor of rat kidney membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50047222

(2-[2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-...)Show SMILES CCC(C)C(NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(=O)NC(C(C)C)C(O)=O Show InChI InChI=1S/C22H33N3O6/c1-5-14(4)19(21(28)23-18(13(2)3)22(29)30)24-20(27)16(12-17(26)25-31)11-15-9-7-6-8-10-15/h6-10,13-14,16,18-19,31H,5,11-12H2,1-4H3,(H,23,28)(H,24,27)(H,25,26)(H,29,30)/t14?,16-,18?,19?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CCIPE-Faculté de Pharmacie
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin converting enzyme |
J Med Chem 36: 1369-79 (1993)
BindingDB Entry DOI: 10.7270/Q2736PZX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50016425

((S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxyca...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
EP CNRS 51
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-BH-CCK- binding to cholecystokinin type B receptor from jurkat Tcells |
J Med Chem 36: 3021-8 (1993)
BindingDB Entry DOI: 10.7270/Q2PZ57VG |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50016425

((S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxyca...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
EP CNRS 51
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-BH-CCK- binding to cholecystokinin type B receptor from guinea pig brain membranes |
J Med Chem 36: 3021-8 (1993)
BindingDB Entry DOI: 10.7270/Q2PZ57VG |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50033631

(2-{2-[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-sul...)Show SMILES CCCC[C@H](NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccc(cc1)S(O)(=O)=O)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)OCCc1ccccc1 Show InChI InChI=1S/C50H65N7O14S2/c1-6-7-16-37(45(62)56-41(28-43(59)60)48(65)70-24-22-31-13-9-8-10-14-31)54-47(64)40(27-33-29-51-36-17-12-11-15-35(33)36)53-42(58)30-52-44(61)38(23-25-72-5)55-46(63)39(57-49(66)71-50(2,3)4)26-32-18-20-34(21-19-32)73(67,68)69/h8-15,17-21,29,37-41,51H,6-7,16,22-28,30H2,1-5H3,(H,52,61)(H,53,58)(H,54,64)(H,55,63)(H,56,62)(H,57,66)(H,59,60)(H,67,68,69)/t37-,38-,39-,40+,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
EP CNRS 51
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of amylase release from rat pancreatic acini |
J Med Chem 36: 3021-8 (1993)
BindingDB Entry DOI: 10.7270/Q2PZ57VG |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50016425

((S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxyca...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Ability of compound to Inhibit the binding of [125I]BH-CCK-8 to Cholecystokinin receptor in isolated guinea pig brain membranes |
J Med Chem 32: 2331-9 (1989)
BindingDB Entry DOI: 10.7270/Q2M61NGS |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50103475

(Pmp-Tyr-Ile-Thr-Asn-Cys-Pro-Orn-phe(I,N3)-NH2 | Pm...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)CC1(CCCCC1)SCCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N1CCCC1C(=O)NC(CCCN)C(=O)NC(Cc1ccc(N=[N+]=[N-])c(I)c1)C(N)=O Show InChI InChI=1S/C57H84IN15O13S2/c1-5-31(2)47(69-52(81)40(26-33-13-16-35(86-4)17-14-33)64-46(77)29-57(20-7-6-8-21-57)88-24-19-44(60)75)54(83)70-48(32(3)74)55(84)67-41(28-45(61)76)51(80)68-42(30-87)56(85)73-23-10-12-43(73)53(82)65-38(11-9-22-59)50(79)66-39(49(62)78)27-34-15-18-37(71-72-63)36(58)25-34/h13-18,25,31-32,38-43,47-48,74,87H,5-12,19-24,26-30,59H2,1-4H3,(H2,60,75)(H2,61,76)(H2,62,78)(H,64,77)(H,65,82)(H,66,79)(H,67,84)(H,68,80)(H,69,81)(H,70,83)/t31-,32+,38?,39?,40-,41-,42-,43?,47-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Ability to inhibit OT-induced inositol phosphate accumulation was determined in CHO cells expressing the human OT receptor |
J Med Chem 44: 3022-30 (2001)
BindingDB Entry DOI: 10.7270/Q29S1Q9F |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50226886
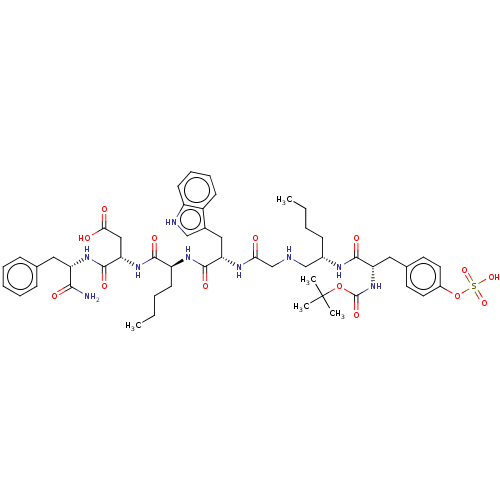
(CHEMBL1159912)Show SMILES CCCC[C@@H](CNCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C52H71N9O14S/c1-6-8-17-35(56-48(67)41(61-51(70)74-52(3,4)5)26-33-21-23-36(24-22-33)75-76(71,72)73)30-54-31-44(62)57-42(27-34-29-55-38-20-14-13-18-37(34)38)49(68)58-39(19-9-7-2)47(66)60-43(28-45(63)64)50(69)59-40(46(53)65)25-32-15-11-10-12-16-32/h10-16,18,20-24,29,35,39-43,54-55H,6-9,17,19,25-28,30-31H2,1-5H3,(H2,53,65)(H,56,67)(H,57,62)(H,58,68)(H,59,69)(H,60,66)(H,61,70)(H,63,64)(H,71,72,73)/t35-,39-,40-,41-,42-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]BH-CCK-9 binding to Cholecystokinin receptor of guinea pig brain membranes |
J Med Chem 30: 1366-73 (1987)
BindingDB Entry DOI: 10.7270/Q2930WDK |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50033632
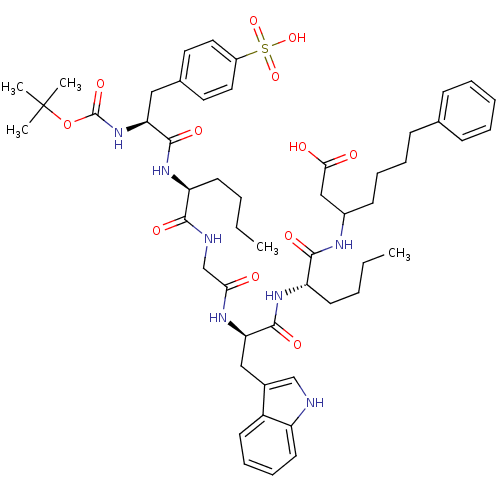
(3-{2-[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-sul...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(cc1)S(O)(=O)=O)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)NC(CCCCc1ccccc1)CC(O)=O Show InChI InChI=1S/C52H71N7O12S/c1-6-8-22-41(57-49(65)43(59-51(67)71-52(3,4)5)29-35-25-27-38(28-26-35)72(68,69)70)47(63)54-33-45(60)56-44(30-36-32-53-40-24-16-15-21-39(36)40)50(66)58-42(23-9-7-2)48(64)55-37(31-46(61)62)20-14-13-19-34-17-11-10-12-18-34/h10-12,15-18,21,24-28,32,37,41-44,53H,6-9,13-14,19-20,22-23,29-31,33H2,1-5H3,(H,54,63)(H,55,64)(H,56,60)(H,57,65)(H,58,66)(H,59,67)(H,61,62)(H,68,69,70)/t37?,41-,42-,43-,44+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
EP CNRS 51
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of amylase release from rat pancreatic acini |
J Med Chem 36: 3021-8 (1993)
BindingDB Entry DOI: 10.7270/Q2PZ57VG |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50226896
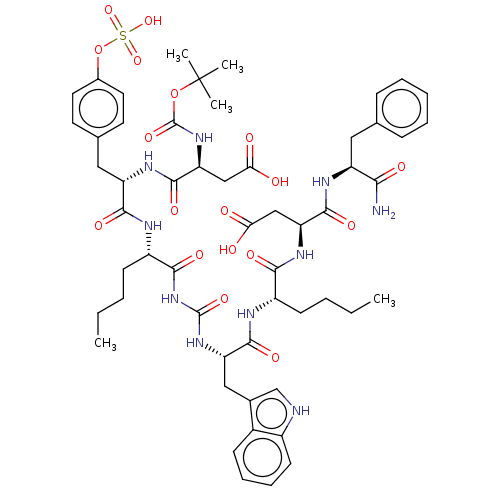
(CHEMBL1159915)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)NC(=O)[C@H](CCCC)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C55H72N10O18S/c1-6-8-18-37(47(71)62-42(28-44(66)67)51(75)60-39(46(56)70)25-31-15-11-10-12-16-31)58-50(74)41(27-33-30-57-36-20-14-13-17-35(33)36)63-53(77)65-48(72)38(19-9-7-2)59-49(73)40(26-32-21-23-34(24-22-32)83-84(79,80)81)61-52(76)43(29-45(68)69)64-54(78)82-55(3,4)5/h10-17,20-24,30,37-43,57H,6-9,18-19,25-29H2,1-5H3,(H2,56,70)(H,58,74)(H,59,73)(H,60,75)(H,61,76)(H,62,71)(H,64,78)(H,66,67)(H,68,69)(H,79,80,81)(H2,63,65,72,77)/t37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]BH-CCK-9 binding to Cholecystokinin receptor of guinea pig brain membranes |
J Med Chem 30: 1366-73 (1987)
BindingDB Entry DOI: 10.7270/Q2930WDK |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50033636
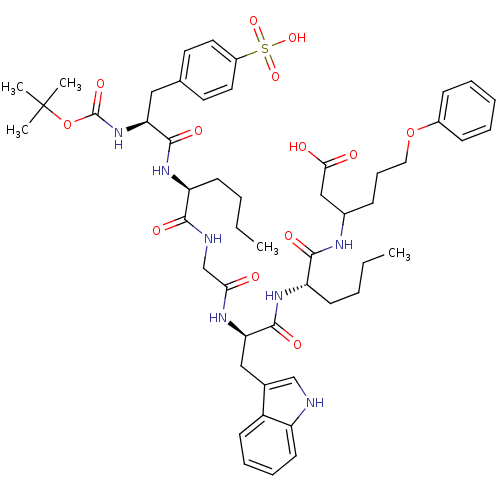
(3-{2-[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-sul...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(cc1)S(O)(=O)=O)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)NC(CCCOc1ccccc1)CC(O)=O Show InChI InChI=1S/C51H69N7O13S/c1-6-8-20-40(56-48(64)42(58-50(66)71-51(3,4)5)28-33-23-25-37(26-24-33)72(67,68)69)46(62)53-32-44(59)55-43(29-34-31-52-39-22-14-13-19-38(34)39)49(65)57-41(21-9-7-2)47(63)54-35(30-45(60)61)16-15-27-70-36-17-11-10-12-18-36/h10-14,17-19,22-26,31,35,40-43,52H,6-9,15-16,20-21,27-30,32H2,1-5H3,(H,53,62)(H,54,63)(H,55,59)(H,56,64)(H,57,65)(H,58,66)(H,60,61)(H,67,68,69)/t35?,40-,41-,42-,43+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EP CNRS 51
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of amylase release from rat pancreatic acini |
J Med Chem 36: 3021-8 (1993)
BindingDB Entry DOI: 10.7270/Q2PZ57VG |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50016425

((S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxyca...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Ability of compound to Inhibit the binding of [125I]BH-CCK-8 to Cholecystokinin receptor in isolated rat pancreatic acini |
J Med Chem 32: 2331-9 (1989)
BindingDB Entry DOI: 10.7270/Q2M61NGS |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50033640

(2-{2-[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-sul...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(cc1)S(O)(=O)=O)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)OCCc1ccccc1 Show InChI InChI=1S/C51H67N7O14S/c1-6-8-18-38(55-47(64)40(58-50(67)72-51(3,4)5)27-33-21-23-35(24-22-33)73(68,69)70)45(62)53-31-43(59)54-41(28-34-30-52-37-20-14-13-17-36(34)37)48(65)56-39(19-9-7-2)46(63)57-42(29-44(60)61)49(66)71-26-25-32-15-11-10-12-16-32/h10-17,20-24,30,38-42,52H,6-9,18-19,25-29,31H2,1-5H3,(H,53,62)(H,54,59)(H,55,64)(H,56,65)(H,57,63)(H,58,67)(H,60,61)(H,68,69,70)/t38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
EP CNRS 51
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-BH-CCK- binding to cholecystokinin type B receptor from jurkat Tcells |
J Med Chem 36: 3021-8 (1993)
BindingDB Entry DOI: 10.7270/Q2PZ57VG |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50033640

(2-{2-[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-sul...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(cc1)S(O)(=O)=O)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)OCCc1ccccc1 Show InChI InChI=1S/C51H67N7O14S/c1-6-8-18-38(55-47(64)40(58-50(67)72-51(3,4)5)27-33-21-23-35(24-22-33)73(68,69)70)45(62)53-31-43(59)54-41(28-34-30-52-37-20-14-13-17-36(34)37)48(65)56-39(19-9-7-2)46(63)57-42(29-44(60)61)49(66)71-26-25-32-15-11-10-12-16-32/h10-17,20-24,30,38-42,52H,6-9,18-19,25-29,31H2,1-5H3,(H,53,62)(H,54,59)(H,55,64)(H,56,65)(H,57,63)(H,58,67)(H,60,61)(H,68,69,70)/t38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
EP CNRS 51
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of amylase release from rat pancreatic acini |
J Med Chem 36: 3021-8 (1993)
BindingDB Entry DOI: 10.7270/Q2PZ57VG |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50227954
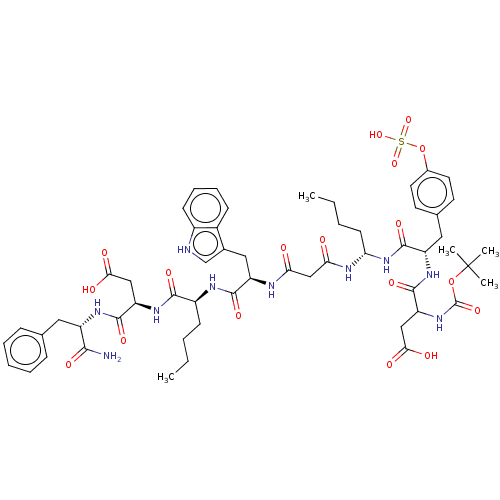
(CHEMBL1160501)Show SMILES CCCC[C@@H](NC(=O)CC(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)C(CC(O)=O)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C56H74N10O18S/c1-6-8-18-38(50(74)63-42(28-47(69)70)52(76)61-39(49(57)73)25-32-15-11-10-12-16-32)60-51(75)41(27-34-31-58-37-19-14-13-17-36(34)37)59-45(67)30-46(68)65-44(20-9-7-2)66-54(78)40(26-33-21-23-35(24-22-33)84-85(80,81)82)62-53(77)43(29-48(71)72)64-55(79)83-56(3,4)5/h10-17,19,21-24,31,38-44,58H,6-9,18,20,25-30H2,1-5H3,(H2,57,73)(H,59,67)(H,60,75)(H,61,76)(H,62,77)(H,63,74)(H,64,79)(H,65,68)(H,66,78)(H,69,70)(H,71,72)(H,80,81,82)/t38-,39-,40-,41+,42+,43?,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Ability of compound to Inhibit the binding of [125I]BH-CCK-8 to Cholecystokinin receptor in isolated guinea pig brain membranes |
J Med Chem 32: 2331-9 (1989)
BindingDB Entry DOI: 10.7270/Q2M61NGS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































