 Found 46 hits with Last Name = 'dastan' and Initial = 'a'
Found 46 hits with Last Name = 'dastan' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50266966
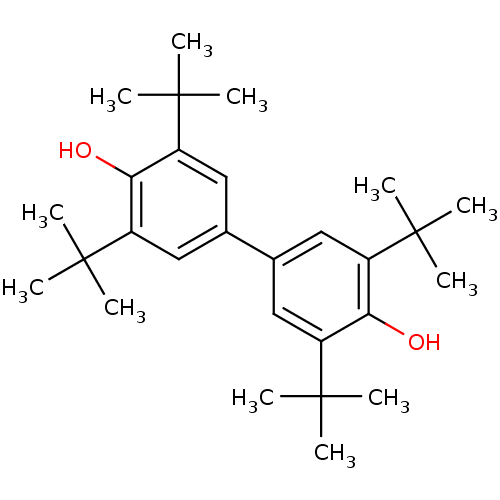
(CHEMBL1662 | di(2,6-di-t-butylphenol))Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C28H42O2/c1-25(2,3)19-13-17(14-20(23(19)29)26(4,5)6)18-15-21(27(7,8)9)24(30)22(16-18)28(10,11)12/h13-16,29-30H,1-12H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50240689
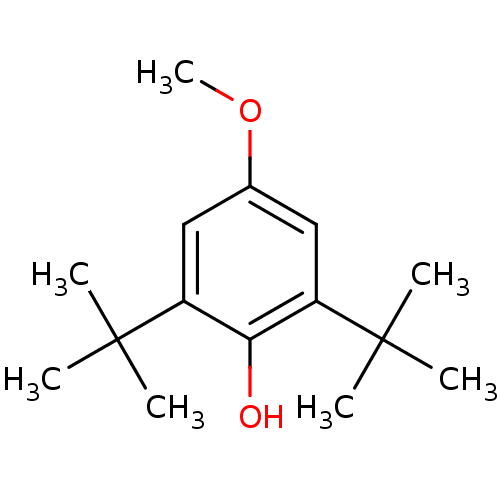
(2,6-Di-tert-butyl-4-methoxy-phenol | 2,6-di-tert-b...)Show InChI InChI=1S/C15H24O2/c1-14(2,3)11-8-10(17-7)9-12(13(11)16)15(4,5)6/h8-9,16H,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50240431

(2,6-Di-tert-butyl-phenol | 2,6-di-t-butylphenol | ...)Show InChI InChI=1S/C14H22O/c1-13(2,3)10-8-7-9-11(12(10)15)14(4,5)6/h7-9,15H,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50079507
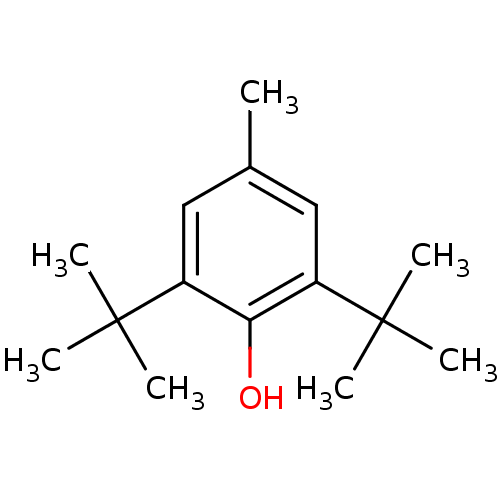
(2,6-Bis(1,1-dimethylethyl)-4-methylphenol | 2,6-Di...)Show InChI InChI=1S/C15H24O/c1-10-8-11(14(2,3)4)13(16)12(9-10)15(5,6)7/h8-9,16H,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50266963
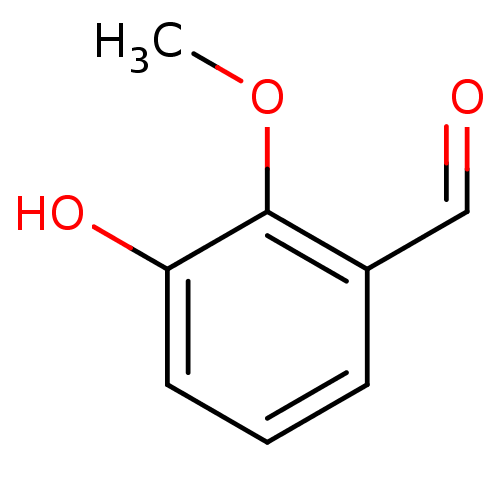
(3-hydroxy-2-methoxybenzaldehyde | CHEMBL507918)Show InChI InChI=1S/C8H8O3/c1-11-8-6(5-9)3-2-4-7(8)10/h2-5,10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50266965
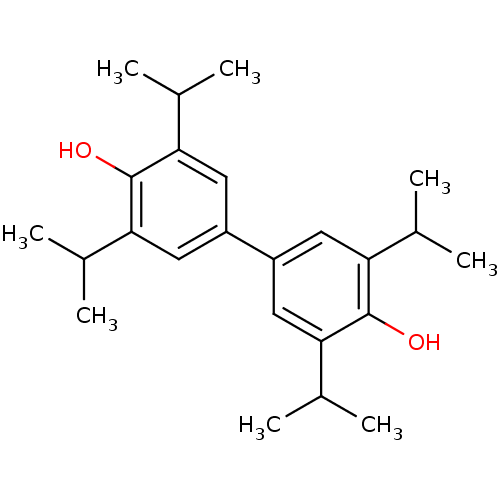
(CHEMBL478518 | di(2,6-diisopropylphenol))Show SMILES CC(C)c1cc(cc(C(C)C)c1O)-c1cc(C(C)C)c(O)c(c1)C(C)C Show InChI InChI=1S/C24H34O2/c1-13(2)19-9-17(10-20(14(3)4)23(19)25)18-11-21(15(5)6)24(26)22(12-18)16(7)8/h9-16,25-26H,1-8H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019398
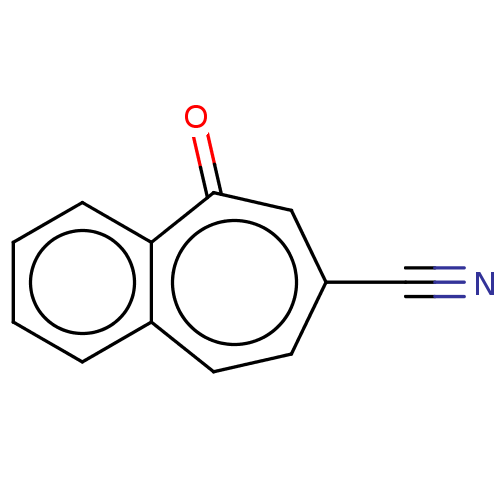
(CHEMBL3290059)Show InChI InChI=1S/C12H7NO/c13-8-9-5-6-10-3-1-2-4-11(10)12(14)7-9/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019395

(CHEMBL3290057)Show InChI InChI=1S/C12H7NO/c13-8-10-6-3-5-9-4-1-2-7-11(9)12(10)14/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019400

(CHEMBL3290055)Show InChI InChI=1S/C12H9N/c13-9-10-4-3-7-11-5-1-2-6-12(11)8-10/h1-6,8H,7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50240369
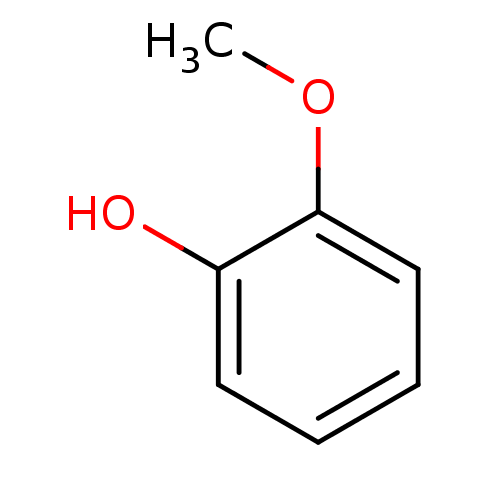
(1-Hydroxy-2-methoxybenzene | 2-Hydroxyanisole | 2-...)Show InChI InChI=1S/C7H8O2/c1-9-7-5-3-2-4-6(7)8/h2-5,8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019400

(CHEMBL3290055)Show InChI InChI=1S/C12H9N/c13-9-10-4-3-7-11-5-1-2-6-12(11)8-10/h1-6,8H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019399
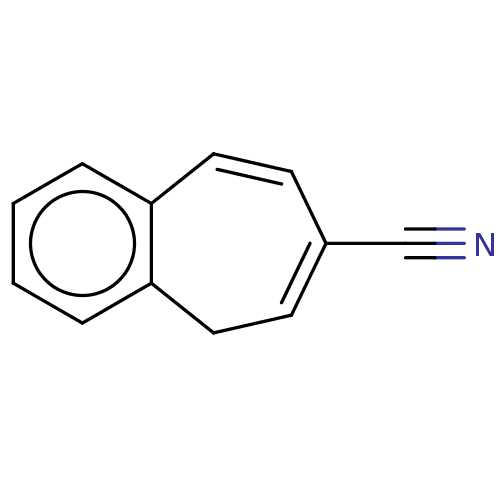
(CHEMBL3290054)Show InChI InChI=1S/C12H9N/c13-9-10-5-7-11-3-1-2-4-12(11)8-6-10/h1-7H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019397
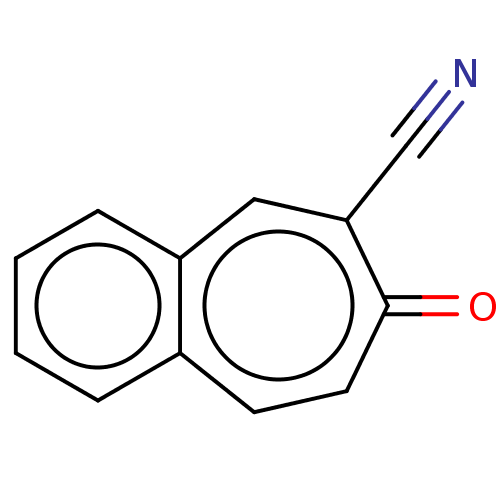
(CHEMBL3290058)Show InChI InChI=1S/C12H7NO/c13-8-11-7-10-4-2-1-3-9(10)5-6-12(11)14/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019398

(CHEMBL3290059)Show InChI InChI=1S/C12H7NO/c13-8-9-5-6-10-3-1-2-4-11(10)12(14)7-9/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019397
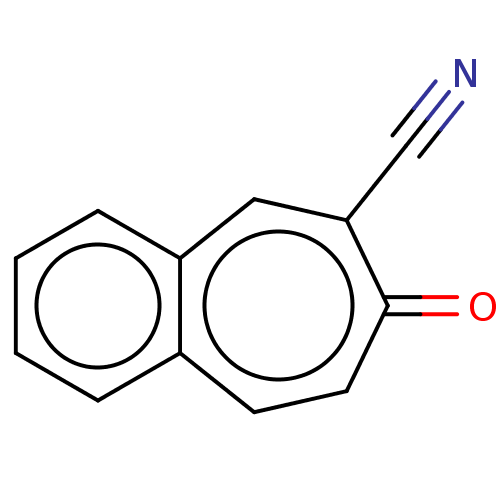
(CHEMBL3290058)Show InChI InChI=1S/C12H7NO/c13-8-11-7-10-4-2-1-3-9(10)5-6-12(11)14/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019394
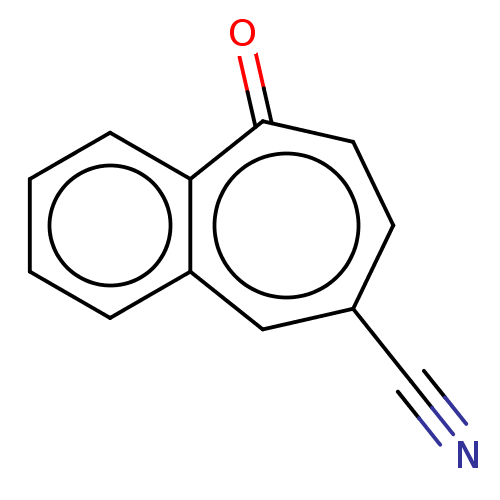
(CHEMBL3290056)Show InChI InChI=1S/C12H7NO/c13-8-9-5-6-12(14)11-4-2-1-3-10(11)7-9/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019394

(CHEMBL3290056)Show InChI InChI=1S/C12H7NO/c13-8-9-5-6-12(14)11-4-2-1-3-10(11)7-9/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019395

(CHEMBL3290057)Show InChI InChI=1S/C12H7NO/c13-8-10-6-3-5-9-4-1-2-7-11(9)12(10)14/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50266964
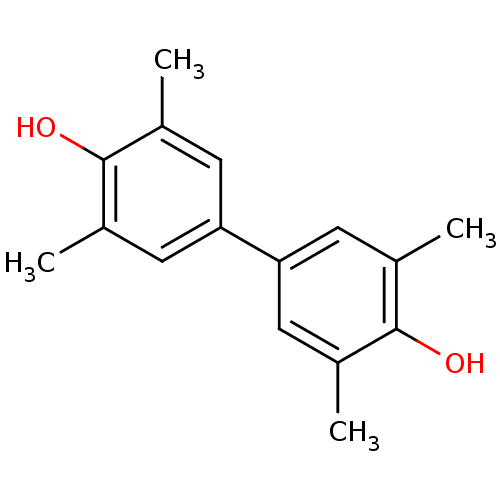
(CHEMBL449983 | di(2,6-dimethylphenol))Show InChI InChI=1S/C16H18O2/c1-9-5-13(6-10(2)15(9)17)14-7-11(3)16(18)12(4)8-14/h5-8,17-18H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019399
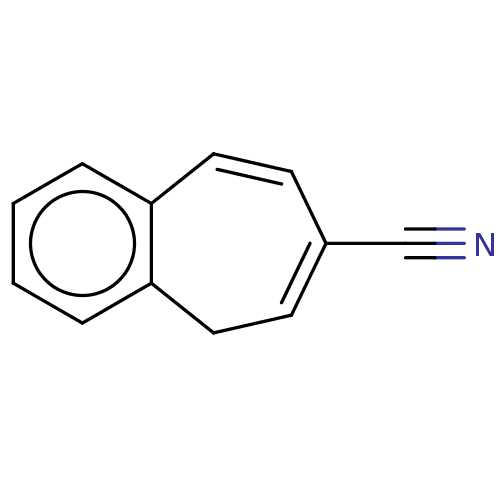
(CHEMBL3290054)Show InChI InChI=1S/C12H9N/c13-9-10-5-7-11-3-1-2-4-12(11)8-6-10/h1-7H,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50058046

(CHEMBL526 | propofol)Show InChI InChI=1S/C12H18O/c1-8(2)10-6-5-7-11(9(3)4)12(10)13/h5-9,13H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50266966

(CHEMBL1662 | di(2,6-di-t-butylphenol))Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C28H42O2/c1-25(2,3)19-13-17(14-20(23(19)29)26(4,5)6)18-15-21(27(7,8)9)24(30)22(16-18)28(10,11)12/h13-16,29-30H,1-12H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50266965

(CHEMBL478518 | di(2,6-diisopropylphenol))Show SMILES CC(C)c1cc(cc(C(C)C)c1O)-c1cc(C(C)C)c(O)c(c1)C(C)C Show InChI InChI=1S/C24H34O2/c1-13(2)19-9-17(10-20(14(3)4)23(19)25)18-11-21(15(5)6)24(26)22(12-18)16(7)8/h9-16,25-26H,1-8H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50266963

(3-hydroxy-2-methoxybenzaldehyde | CHEMBL507918)Show InChI InChI=1S/C8H8O3/c1-11-8-6(5-9)3-2-4-7(8)10/h2-5,10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50240369

(1-Hydroxy-2-methoxybenzene | 2-Hydroxyanisole | 2-...)Show InChI InChI=1S/C7H8O2/c1-9-7-5-3-2-4-6(7)8/h2-5,8H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50240689

(2,6-Di-tert-butyl-4-methoxy-phenol | 2,6-di-tert-b...)Show InChI InChI=1S/C15H24O2/c1-14(2,3)11-8-10(17-7)9-12(13(11)16)15(4,5)6/h8-9,16H,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50266964

(CHEMBL449983 | di(2,6-dimethylphenol))Show InChI InChI=1S/C16H18O2/c1-9-5-13(6-10(2)15(9)17)14-7-11(3)16(18)12(4)8-14/h5-8,17-18H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50058046

(CHEMBL526 | propofol)Show InChI InChI=1S/C12H18O/c1-8(2)10-6-5-7-11(9(3)4)12(10)13/h5-9,13H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50240430

(2,6-Dimethyl phenol | 2,6-Dimethyl-phenol | 2,6-Di...)Show InChI InChI=1S/C8H10O/c1-6-4-3-5-7(2)8(6)9/h3-5,9H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA2 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50240430

(2,6-Dimethyl phenol | 2,6-Dimethyl-phenol | 2,6-Di...)Show InChI InChI=1S/C8H10O/c1-6-4-3-5-7(2)8(6)9/h3-5,9H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50079507

(2,6-Bis(1,1-dimethylethyl)-4-methylphenol | 2,6-Di...)Show InChI InChI=1S/C15H24O/c1-10-8-11(14(2,3)4)13(16)12(9-10)15(5,6)7/h8-9,16H,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50240431

(2,6-Di-tert-butyl-phenol | 2,6-di-t-butylphenol | ...)Show InChI InChI=1S/C14H22O/c1-13(2,3)10-8-7-9-11(12(10)15)14(4,5)6/h7-9,15H,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte CA1 esterase activity using 4-nitrophenyl acetate substrate |
Bioorg Med Chem 17: 3207-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.067
BindingDB Entry DOI: 10.7270/Q2N58M7V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019397
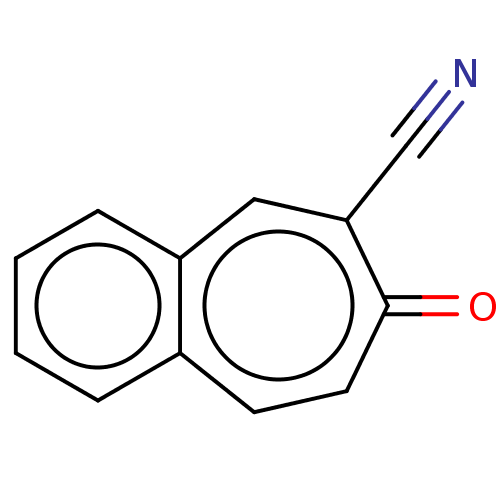
(CHEMBL3290058)Show InChI InChI=1S/C12H7NO/c13-8-11-7-10-4-2-1-3-9(10)5-6-12(11)14/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019395

(CHEMBL3290057)Show InChI InChI=1S/C12H7NO/c13-8-10-6-3-5-9-4-1-2-7-11(9)12(10)14/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019398

(CHEMBL3290059)Show InChI InChI=1S/C12H7NO/c13-8-9-5-6-10-3-1-2-4-11(10)12(14)7-9/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019394

(CHEMBL3290056)Show InChI InChI=1S/C12H7NO/c13-8-9-5-6-12(14)11-4-2-1-3-10(11)7-9/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019400

(CHEMBL3290055)Show InChI InChI=1S/C12H9N/c13-9-10-4-3-7-11-5-1-2-6-12(11)8-10/h1-6,8H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50019399
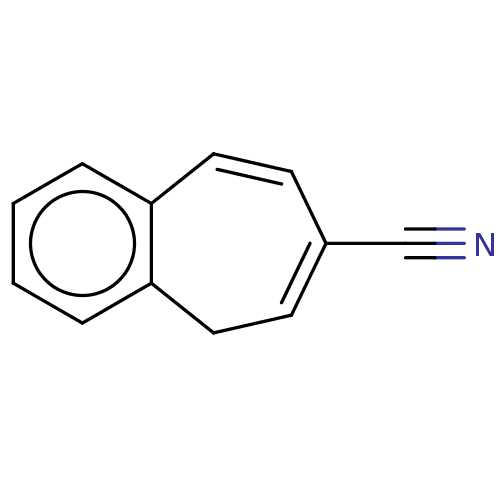
(CHEMBL3290054)Show InChI InChI=1S/C12H9N/c13-9-10-5-7-11-3-1-2-4-12(11)8-6-10/h1-7H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-2 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019399
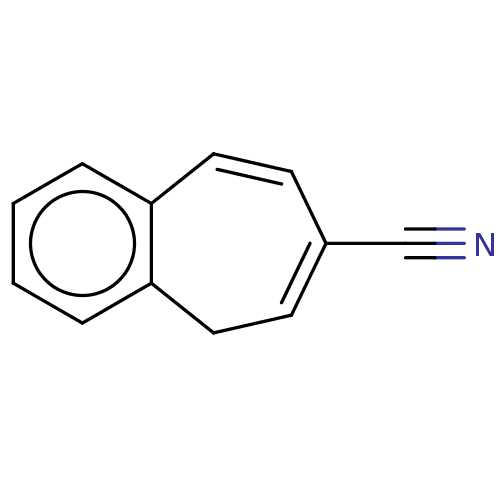
(CHEMBL3290054)Show InChI InChI=1S/C12H9N/c13-9-10-5-7-11-3-1-2-4-12(11)8-6-10/h1-7H,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019398

(CHEMBL3290059)Show InChI InChI=1S/C12H7NO/c13-8-9-5-6-10-3-1-2-4-11(10)12(14)7-9/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019397
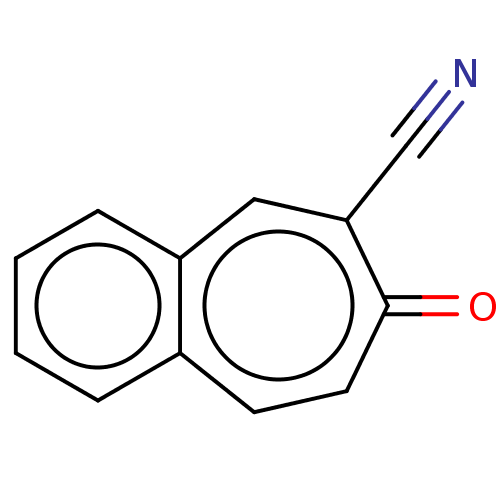
(CHEMBL3290058)Show InChI InChI=1S/C12H7NO/c13-8-11-7-10-4-2-1-3-9(10)5-6-12(11)14/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019400

(CHEMBL3290055)Show InChI InChI=1S/C12H9N/c13-9-10-4-3-7-11-5-1-2-6-12(11)8-10/h1-6,8H,7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019394

(CHEMBL3290056)Show InChI InChI=1S/C12H7NO/c13-8-9-5-6-12(14)11-4-2-1-3-10(11)7-9/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50019395

(CHEMBL3290057)Show InChI InChI=1S/C12H7NO/c13-8-10-6-3-5-9-4-1-2-7-11(9)12(10)14/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic CA-1 assessed as p-nitrophenolate formation after 3 mins using p-nitrophenylacetate as substrate by spectrophotometer |
Bioorg Med Chem 22: 3537-43 (2014)
Article DOI: 10.1016/j.bmc.2014.04.007
BindingDB Entry DOI: 10.7270/Q2Z60QM4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data