

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239101 (US10195181, Example 2.2 | US9403810, 2.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239101 (US10195181, Example 2.2 | US9403810, 2.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239132 (US9403810, 22c) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239117 (US10195181, Example 22c | US9403810, 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239094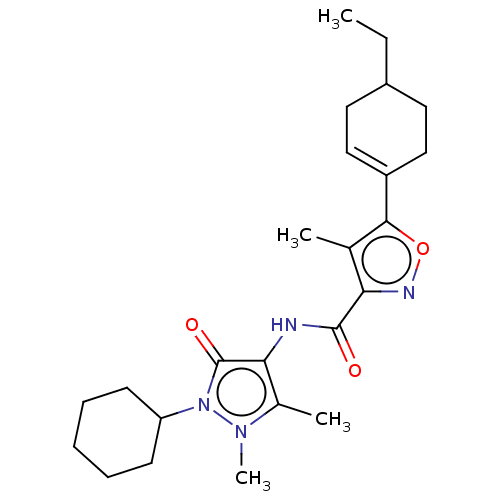 (US10195181, Example 1.3 | US9403810, 1.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239094 (US10195181, Example 1.3 | US9403810, 1.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528195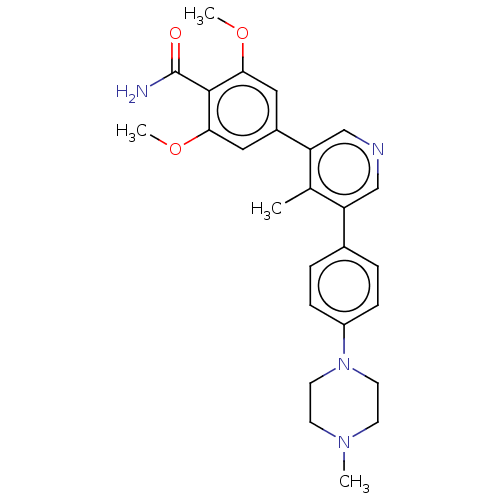 (CHEMBL4435320) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of ALK2 G328V mutant (unknown origin) | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528203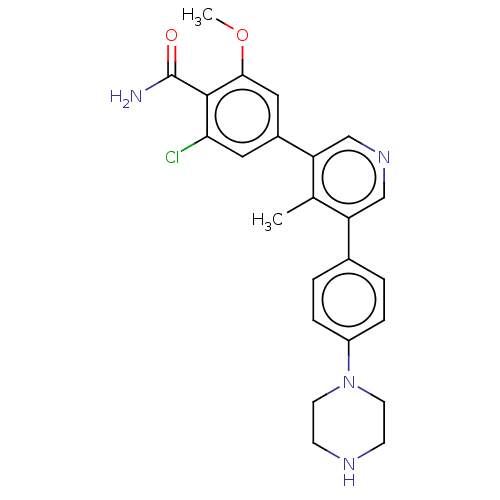 (CHEMBL4434694) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of ALK2 G328V mutant (unknown origin) | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528195 (CHEMBL4435320) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of ALK2 R258G mutant (unknown origin) | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239092 (US10195181, Example 1.1 | US9403810, 1.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239092 (US10195181, Example 1.1 | US9403810, 1.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate kinase 3 (Homo sapiens (Human)) | BDBM649476 (US11891378, Example 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239099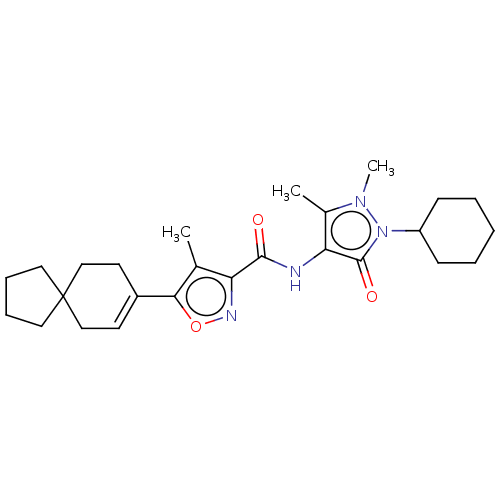 (US10195181, Example 2 | US9403810, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239099 (US10195181, Example 2 | US9403810, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239091 (US10195181, Example 1 | US9403810, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239091 (US10195181, Example 1 | US9403810, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate kinase 3 (Homo sapiens (Human)) | BDBM649472 (US11891378, Example 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528195 (CHEMBL4435320) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544399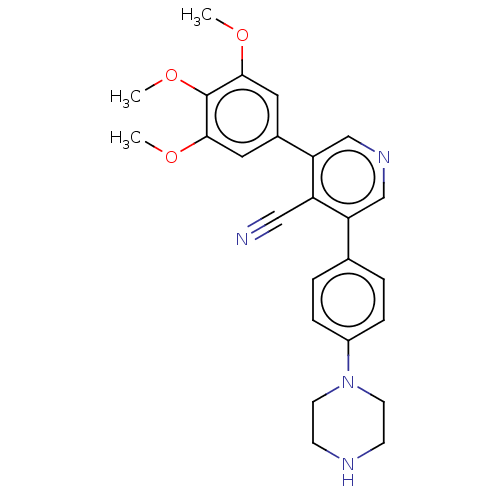 (CHEMBL4638273) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528196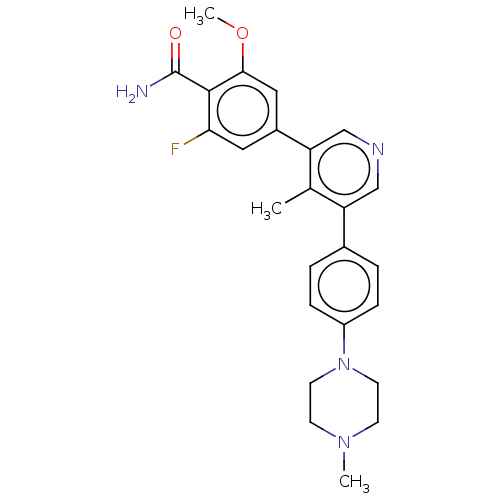 (CHEMBL4575655) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528190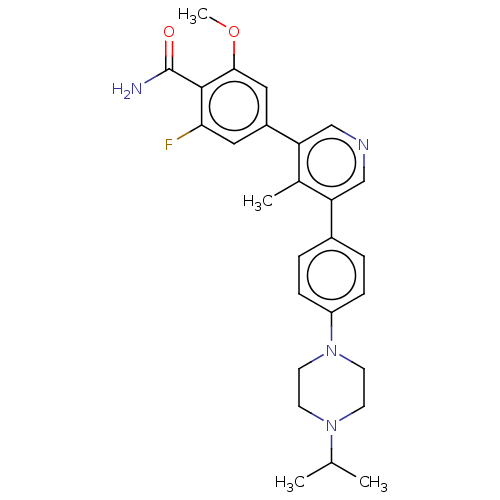 (CHEMBL4548795) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of ALK2 G328V mutant (unknown origin) | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528209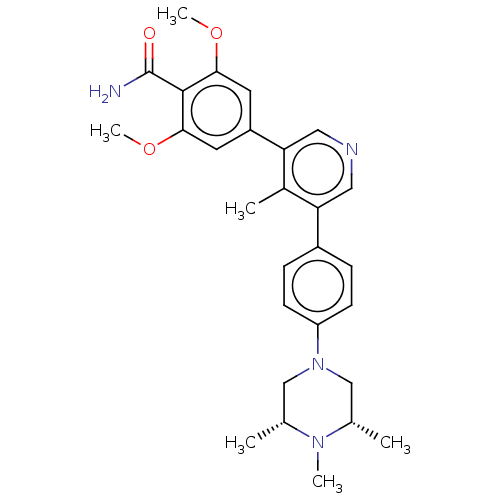 (CHEMBL4526828) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239095 (US10195181, Example 1.4 | US9403810, 1.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239095 (US10195181, Example 1.4 | US9403810, 1.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525146 (US11168102, Example 32-31.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate kinase 3 (Homo sapiens (Human)) | BDBM649480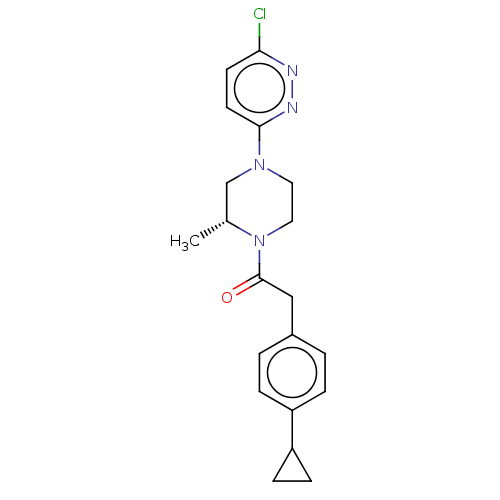 (US11891378, Example 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528195 (CHEMBL4435320) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 R206H mutant using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528203 (CHEMBL4434694) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of ALK2 R258G mutant (unknown origin) | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528208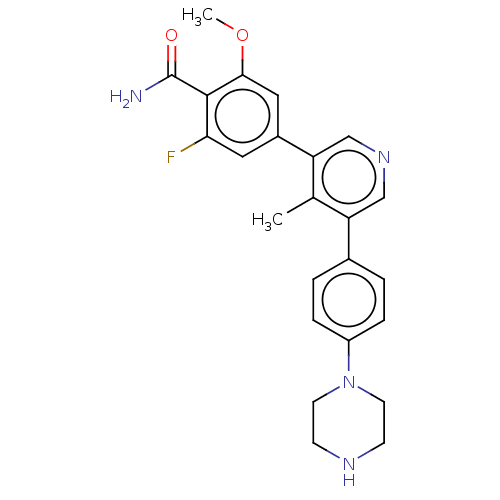 (CHEMBL4440168) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of ALK2 G328V mutant (unknown origin) | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544408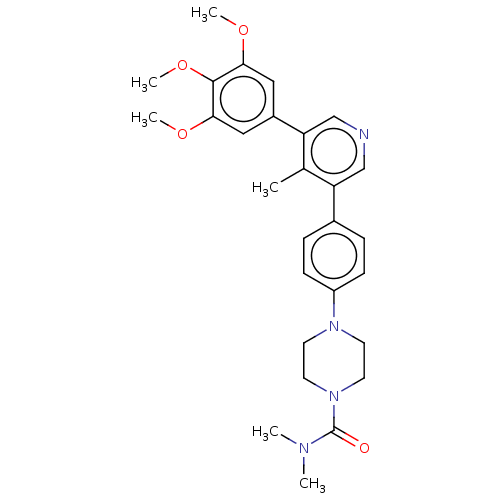 (CHEMBL4641207) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525147 (US11168102, Example 32-32.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528203 (CHEMBL4434694) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 R206H mutant using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544414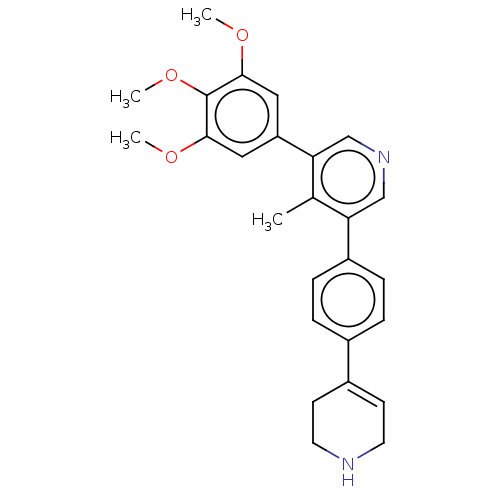 (CHEMBL4641530) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528204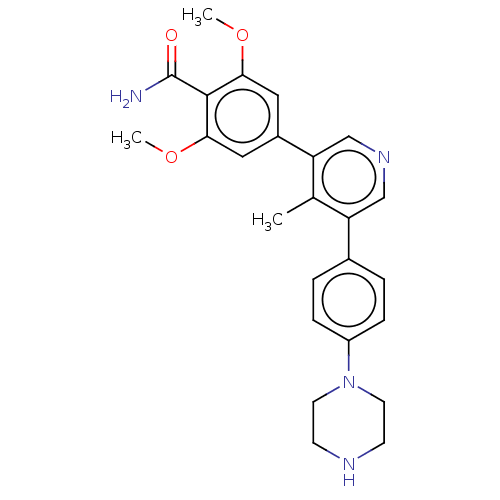 (CHEMBL4565968) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528190 (CHEMBL4548795) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528188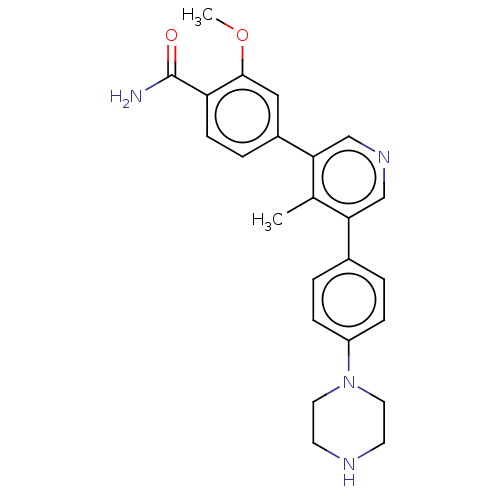 (CHEMBL4553210) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of ALK2 G328V mutant (unknown origin) | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528205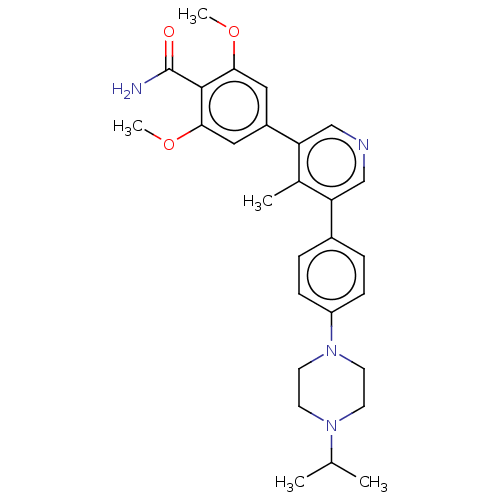 (CHEMBL4468389) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate kinase 3 (Homo sapiens (Human)) | BDBM649479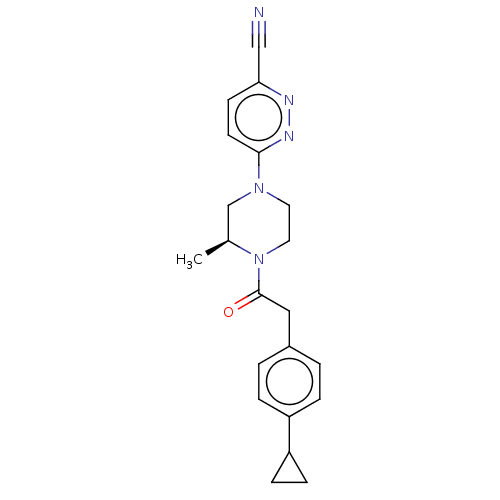 (US11891378, Example 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239105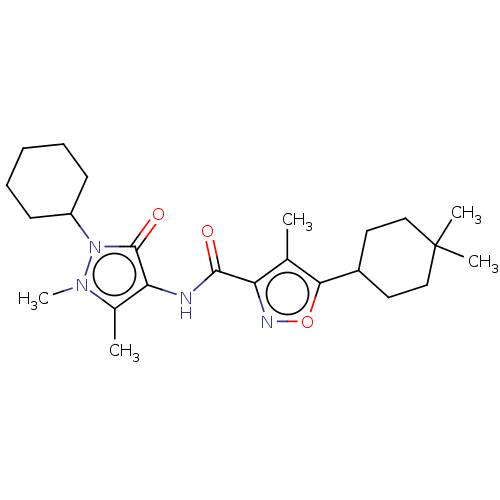 (US10195181, Example 6 | US9403810, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239105 (US10195181, Example 6 | US9403810, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525070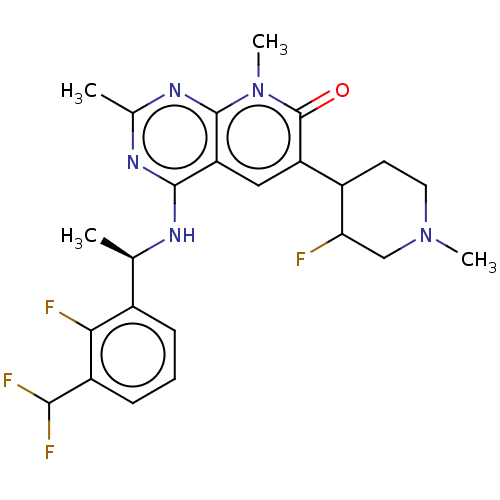 (US11168102, Example 18.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate kinase 3 (Homo sapiens (Human)) | BDBM649477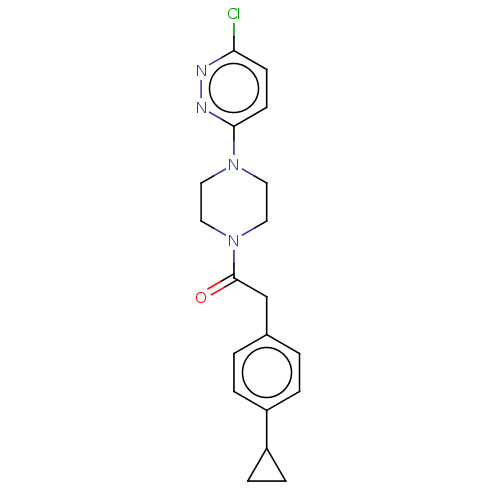 (US11891378, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544419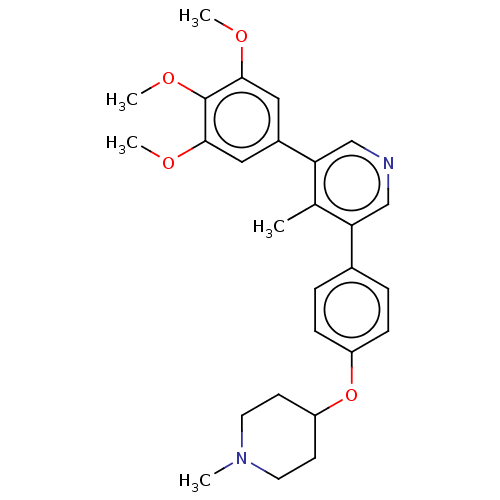 (CHEMBL4647724) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528190 (CHEMBL4548795) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 R206H mutant using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239102 (US10195181, Example 3 | US9403810, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528194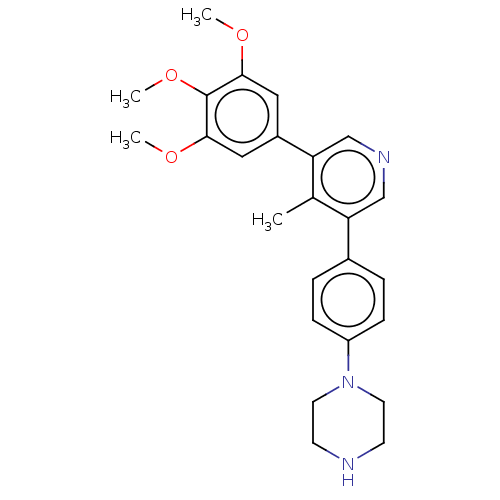 (CHEMBL4517408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Competitive displacement of PBI-6908 from nanoluciferase-fused ALK2 G328V mutant (unknown origin) expressed in HEK293 cells incubated for 2 hrs by Na... | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50544418 (CHEMBL4648102) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay | J Med Chem 63: 10061-10085 (2020) Article DOI: 10.1021/acs.jmedchem.0c01199 BindingDB Entry DOI: 10.7270/Q2VM4GV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528190 (CHEMBL4548795) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of ALK2 R258G mutant (unknown origin) | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 BindingDB Entry DOI: 10.7270/Q2MK6HB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239096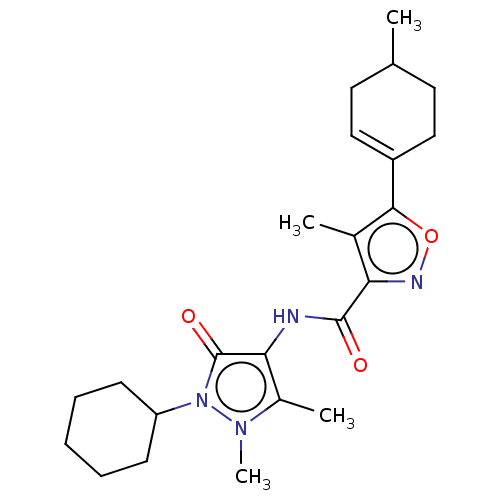 (US10195181, Example 1.5 | US9403810, 1.5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239102 (US10195181, Example 3 | US9403810, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 702 total ) | Next | Last >> |