

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14722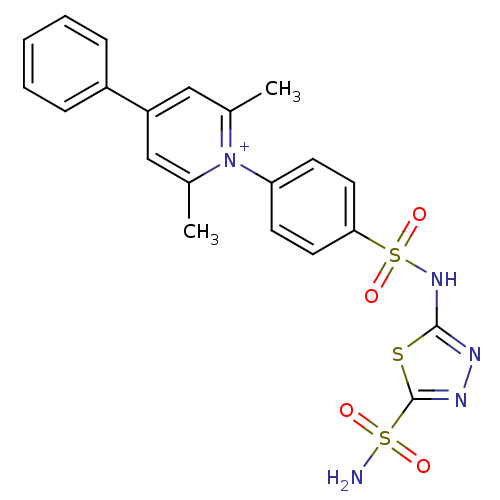 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14723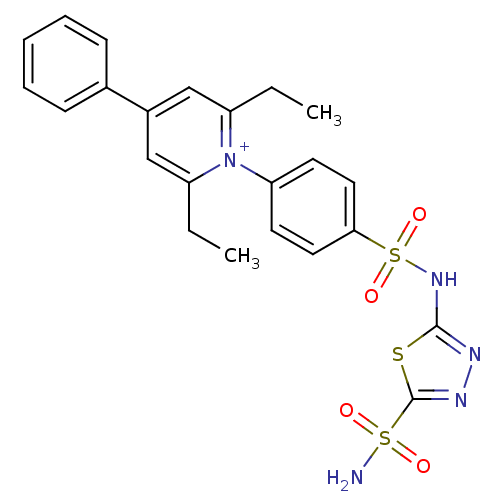 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14718 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10860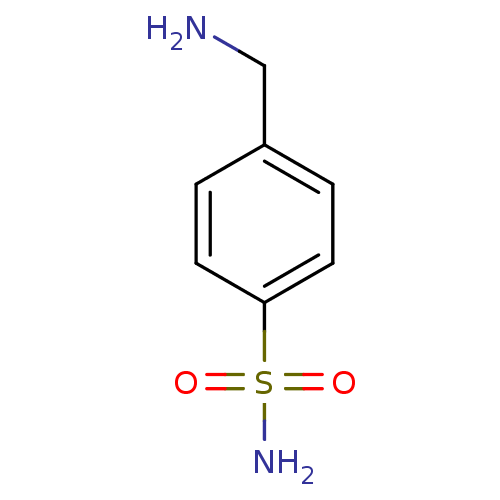 (4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14736 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14719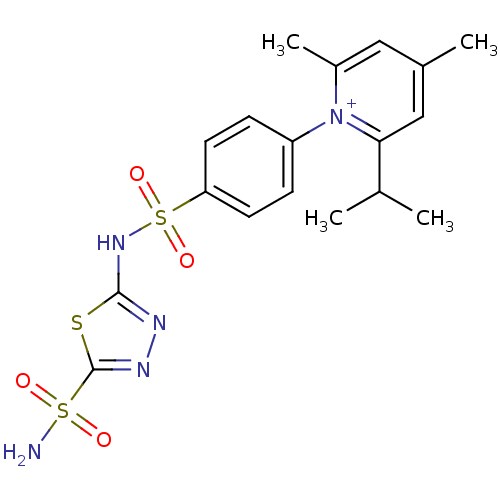 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10862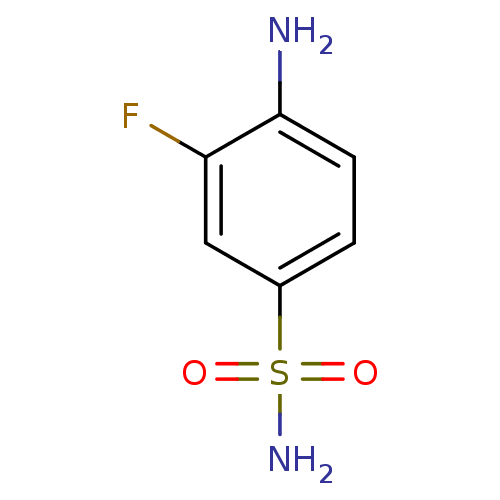 (4-Amino-3-fluorobenzenesulfonamide | 4-amino-3-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14728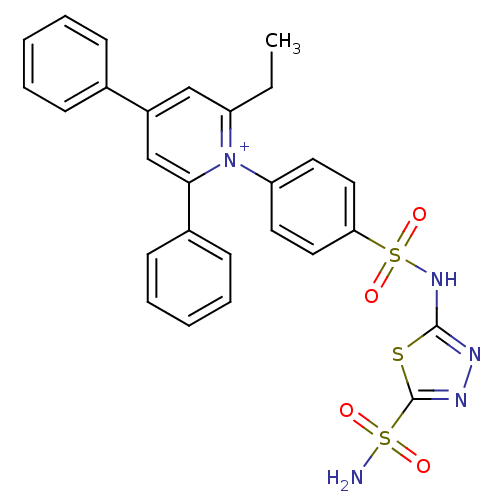 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14727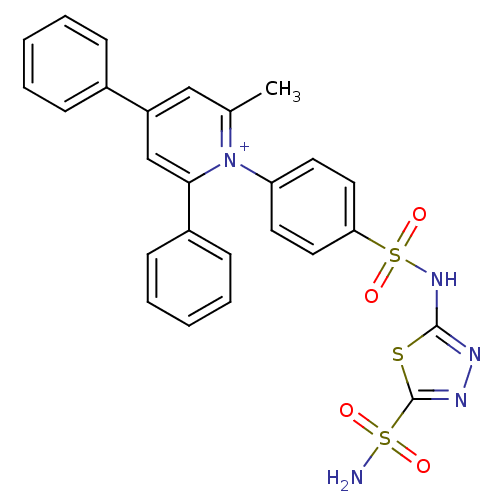 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14737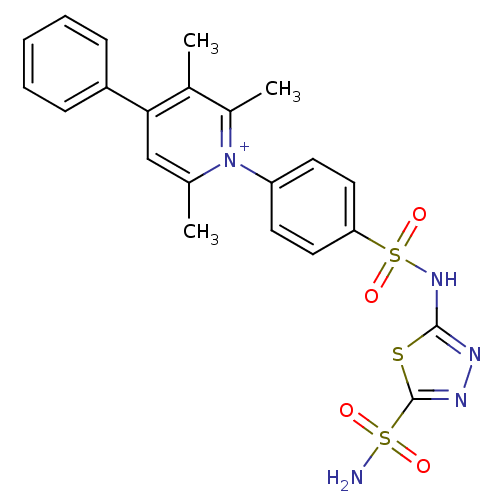 (2,3,6-trimethyl-4-phenyl-1-{4-[(5-sulfamoyl-1,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50098106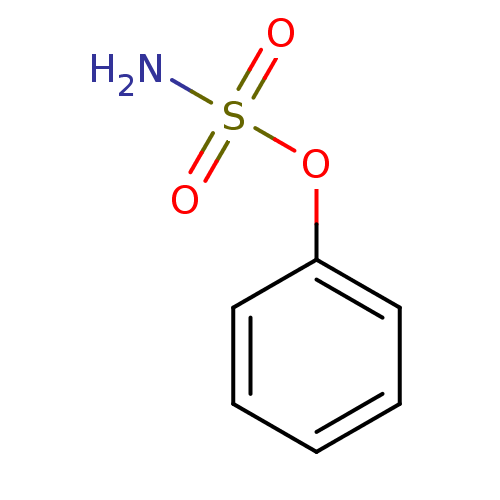 (CHEMBL24259 | PHENYLSULFAMATE | Sulfamic acid phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Union Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 preincubated for 15 mins measured for 10 to 100 sec by stopped-flow method | J Med Chem 55: 3513-20 (2012) Article DOI: 10.1021/jm300203r BindingDB Entry DOI: 10.7270/Q2RX9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50098108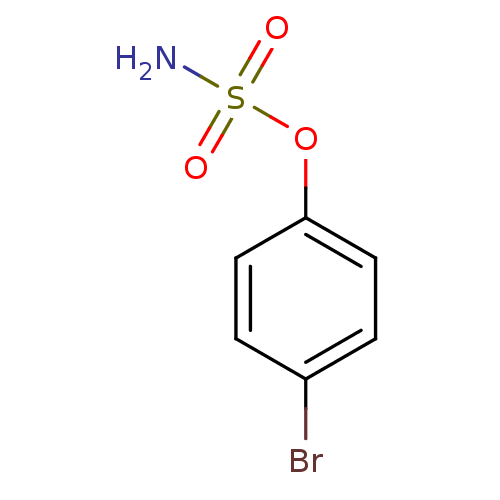 (CHEMBL283121 | Sulfamic acid 4-bromo-phenyl ester) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Union Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 preincubated for 15 mins measured for 10 to 100 sec by stopped-flow method | J Med Chem 55: 3513-20 (2012) Article DOI: 10.1021/jm300203r BindingDB Entry DOI: 10.7270/Q2RX9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14720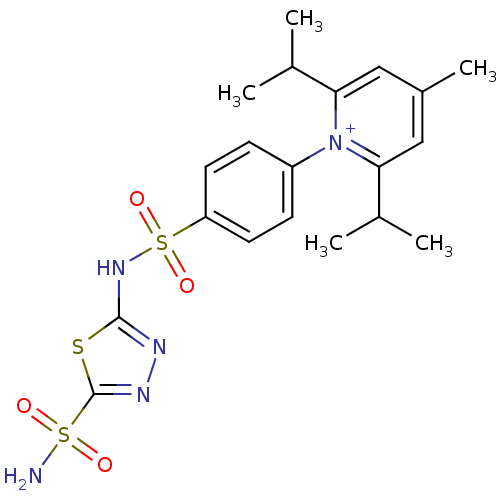 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14726 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10870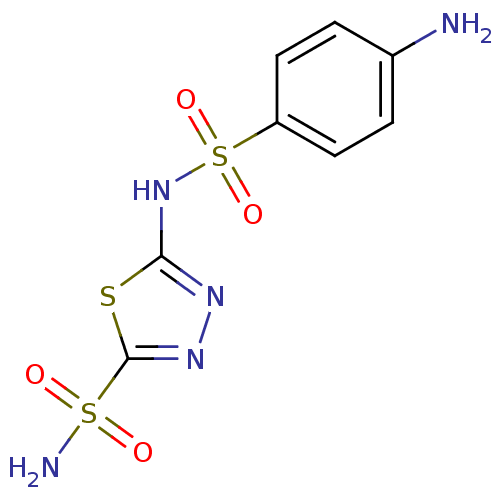 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Union Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 preincubated for 15 mins measured for 10 to 100 sec by stopped-flow method | J Med Chem 55: 3513-20 (2012) Article DOI: 10.1021/jm300203r BindingDB Entry DOI: 10.7270/Q2RX9D4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14722 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 7860-6 (2005) Article DOI: 10.1021/jm050483n BindingDB Entry DOI: 10.7270/Q2902200 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14718 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14723 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14736 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM14737 (2,3,6-trimethyl-4-phenyl-1-{4-[(5-sulfamoyl-1,3,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10885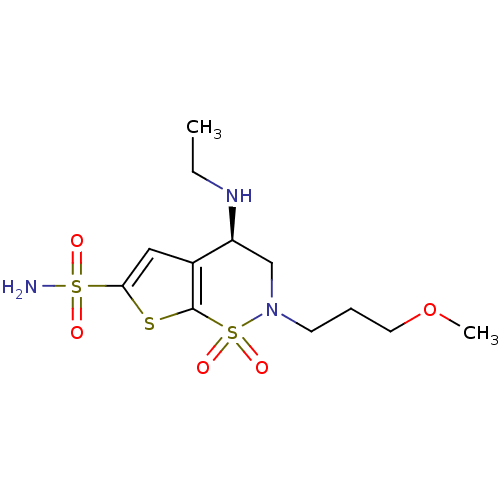 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 7860-6 (2005) Article DOI: 10.1021/jm050483n BindingDB Entry DOI: 10.7270/Q2902200 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM14718 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14727 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14719 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM14722 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM14736 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Union Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 preincubated for 15 mins measured for 10 to 100 sec by stopped-flow method | J Med Chem 55: 3513-20 (2012) Article DOI: 10.1021/jm300203r BindingDB Entry DOI: 10.7270/Q2RX9D4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10863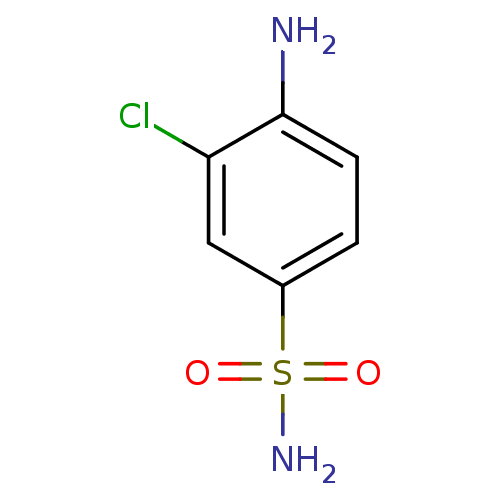 (4-Amino-3-chlorobenzenesulfonamide | 4-amino-3-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14721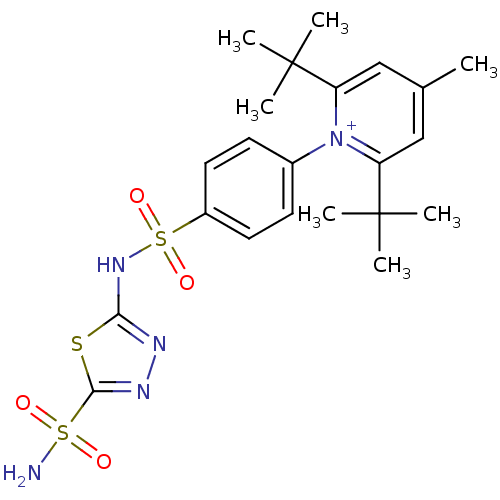 (2,6-di-tert-butyl-4-methyl-1-{4-[(5-sulfamoyl-1,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10861 (4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10881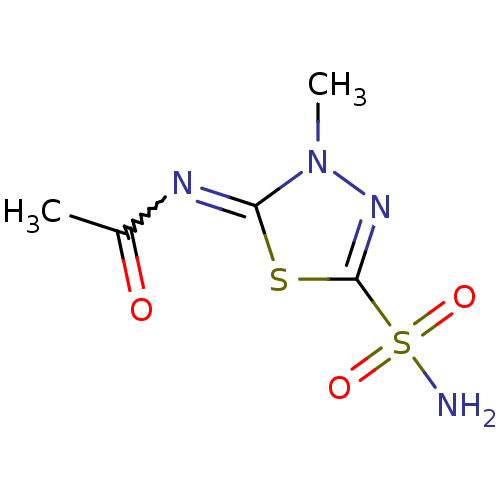 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10873 (4-[(2-aminopyrimidin-4-yl)amino]benzene-1-sulfonam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14724 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10886 (2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10874 (6-hydroxy-1,3-benzothiazole-2-sulfonamide | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14730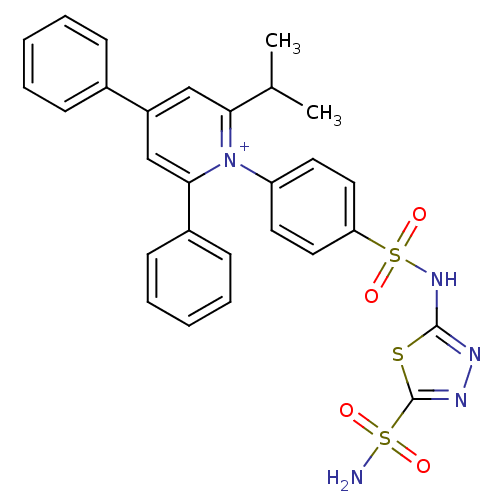 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14728 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14729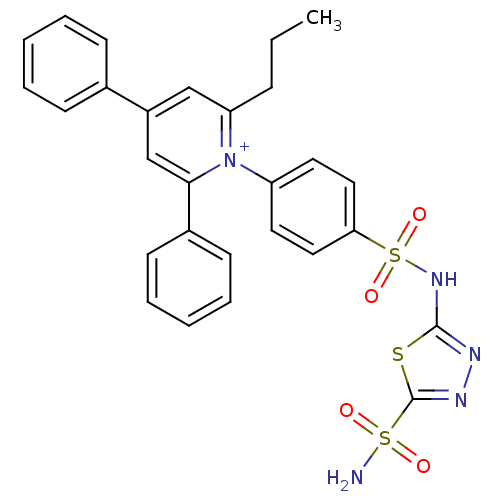 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM14719 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM14727 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM14718 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM14723 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10869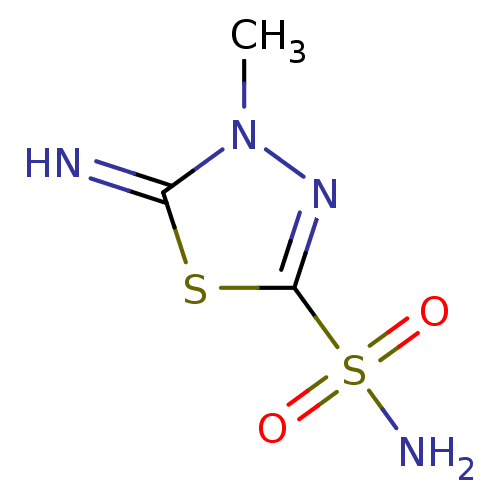 (5-imino-4-methyl-4,5-dihydro-1,3,4-thiadiazole-2-s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibitory constant against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3828-33 (2005) Article DOI: 10.1016/j.bmcl.2005.06.055 BindingDB Entry DOI: 10.7270/Q2C82B2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14726 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM14732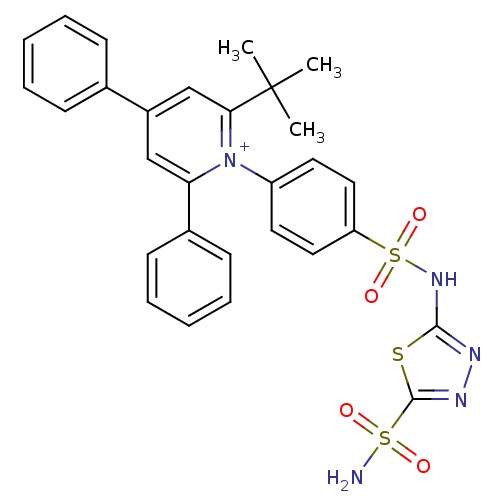 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14730 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 414 total ) | Next | Last >> |