 Found 78 hits with Last Name = 'yamashita' and Initial = 'a'
Found 78 hits with Last Name = 'yamashita' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290098

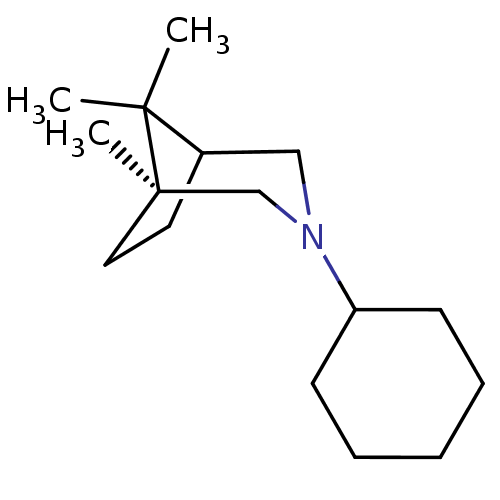
((R)-3-Cyclohexylmethyl-1,8,8-trimethyl-3-aza-bicyc...)Show SMILES CC1(C)C2CC[C@@]1(C)CN(CC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C17H31N/c1-16(2)15-9-10-17(16,3)13-18(12-15)11-14-7-5-4-6-8-14/h14-15H,4-13H2,1-3H3/t15?,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290099

((S)-3-(4-Cyclohexyl-butyl)-1,8,8-trimethyl-3-aza-b...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCCCC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C20H37N/c1-19(2)18-12-13-20(19,3)16-21(15-18)14-8-7-11-17-9-5-4-6-10-17/h17-18H,4-16H2,1-3H3/t18?,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290100

((S)-3-Cyclohexylmethyl-1,8,8-trimethyl-3-aza-bicyc...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C17H31N/c1-16(2)15-9-10-17(16,3)13-18(12-15)11-14-7-5-4-6-8-14/h14-15H,4-13H2,1-3H3/t15?,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290095

((R)-3-(2-Cyclohexyl-ethyl)-1,8,8-trimethyl-3-aza-b...)Show SMILES CC1(C)C2CC[C@@]1(C)CN(CCC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C18H33N/c1-17(2)16-9-11-18(17,3)14-19(13-16)12-10-15-7-5-4-6-8-15/h15-16H,4-14H2,1-3H3/t16?,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290096

((S)-3-(2-Cyclopentyl-ethyl)-1,8,8-trimethyl-3-aza-...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1CCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C17H31N/c1-16(2)15-8-10-17(16,3)13-18(12-15)11-9-14-6-4-5-7-14/h14-15H,4-13H2,1-3H3/t15?,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290094

((S)-3-(2-Cyclohexyl-ethyl)-1,8,8-trimethyl-3-aza-b...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C18H33N/c1-17(2)16-9-11-18(17,3)14-19(13-16)12-10-15-7-5-4-6-8-15/h15-16H,4-14H2,1-3H3/t16?,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290101

((S)-3-(3-Cyclohexyl-propyl)-1,8,8-trimethyl-3-aza-...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCCC1CCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C19H35N/c1-18(2)17-11-12-19(18,3)15-20(14-17)13-7-10-16-8-5-4-6-9-16/h16-17H,4-15H2,1-3H3/t17?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290093

((S)-3-(2-Cyclooctyl-ethyl)-1,8,8-trimethyl-3-aza-b...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1CCCCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C20H37N/c1-19(2)18-11-13-20(19,3)16-21(15-18)14-12-17-9-7-5-4-6-8-10-17/h17-18H,4-16H2,1-3H3/t18?,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290092

((S)-3-(2-Cycloheptyl-ethyl)-1,8,8-trimethyl-3-aza-...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1CCCCCC1)C2 |THB:10:9:1:5.4| Show InChI InChI=1S/C19H35N/c1-18(2)17-10-12-19(18,3)15-20(14-17)13-11-16-8-6-4-5-7-9-16/h16-17H,4-15H2,1-3H3/t17?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290097
((S)-3-Cyclohexyl-1,8,8-trimethyl-3-aza-bicyclo[3.2...)Show SMILES CC1(C)C2CC[C@]1(C)CN(C2)C1CCCCC1 |THB:11:9:1:5.4| Show InChI InChI=1S/C16H29N/c1-15(2)13-9-10-16(15,3)12-17(11-13)14-7-5-4-6-8-14/h13-14H,4-12H2,1-3H3/t13?,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50290091

((S)-3-(2-Adamantan-2-yl-ethyl)-1,8,8-trimethyl-3-a...)Show SMILES CC1(C)C2CC[C@]1(C)CN(CCC1C3CC4CC(C3)CC1C4)C2 |wD:6.7,TLB:11:12:14:17.18.16,THB:16:15:12:17.18.19,16:17:12:15.14.21,10:9:1:5.4,11:12:15.14.21:17.18.19,19:20:14:17.18.16,19:17:14:20.12.21,(-7.25,2.8,;-5.75,2.79,;-6.14,4.25,;-4.39,2.21,;-4,.76,;-4.93,-.34,;-4.93,1.16,;-6.43,1.16,;-2.69,2.13,;-1.35,1.44,;-.27,.4,;1.17,.82,;2.25,-.23,;2.88,-1.98,;2.25,-3.46,;3.36,-4.49,;5,-4.47,;5.66,-2.93,;4.41,-2.03,;5.18,-1.24,;3.56,-1.24,;2.87,-2.78,;-2.32,3.17,)| Show InChI InChI=1S/C22H37N/c1-21(2)19-4-6-22(21,3)14-23(13-19)7-5-20-17-9-15-8-16(11-17)12-18(20)10-15/h15-20H,4-14H2,1-3H3/t15?,16?,17?,18?,19?,20?,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM82213
(CAS_41598-07-6 | NSC_114678 | PGD2)Show SMILES CCCCCC(O)CC=C1C(CC=CCCCC(O)=O)C=CC1=O |w:8.7,12.11,c:20| Show InChI InChI=1S/C20H30O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,14-17,21H,2-3,5-6,8-11,13H2,1H3,(H,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM82214

(CAS_114676 | NSC_114676 | PGD2-methyl ester)Show SMILES CCCCCC(O)C=CC1C(CC=CCCCC(=O)OC)C(O)CC1=O |w:7.6,12.11| Show InChI InChI=1S/C21H34O5/c1-3-4-7-10-16(22)13-14-18-17(19(23)15-20(18)24)11-8-5-6-9-12-21(25)26-2/h5,8,13-14,16-19,22-23H,3-4,6-7,9-12,15H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM81982
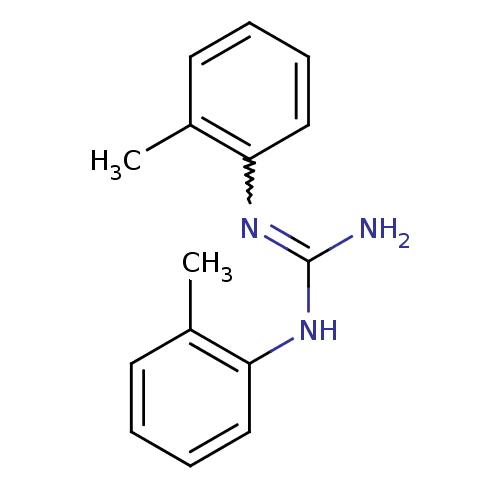
(CAS_97-39-2 | DITOLYLGUANIDINE | DTG | Di-o-tolylg...)Show InChI InChI=1S/C15H17N3/c1-11-7-3-5-9-13(11)17-15(16)18-14-10-6-4-8-12(14)2/h3-10H,1-2H3,(H3,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50001044

((1S,9S,13S)-10-allyl-1,13-dimethyl-10-azatricyclo[...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pentazocine binding to Sigma opioid receptor type 1 |
Bioorg Med Chem Lett 7: 2303-2306 (1997)
Article DOI: 10.1016/S0960-894X(97)00417-4
BindingDB Entry DOI: 10.7270/Q2319VVV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM82209

(CAS_205449 | NSC_205449 | PGD1)Show SMILES CCCCCC(O)C=CC1C(CCCCCCC(O)=O)C(O)CC1=O |w:7.6| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-18,21-22H,2-11,14H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 523 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM50020300
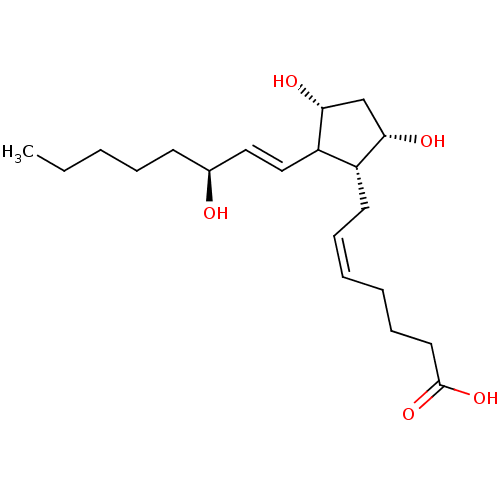
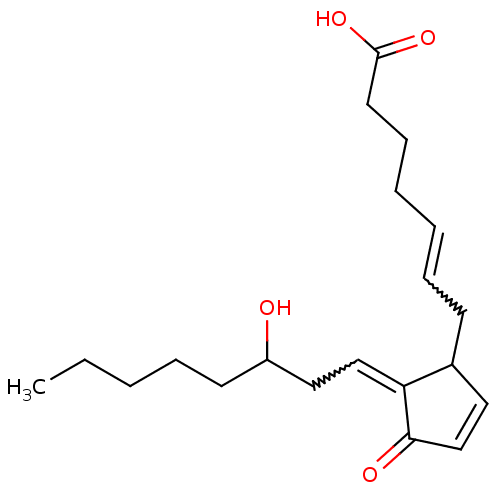
((S-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-oct-1-enyl...)Show SMILES CCCCC[C@H](O)\C=C\C1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-19,21-23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17?,18-,19+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 693 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM82215
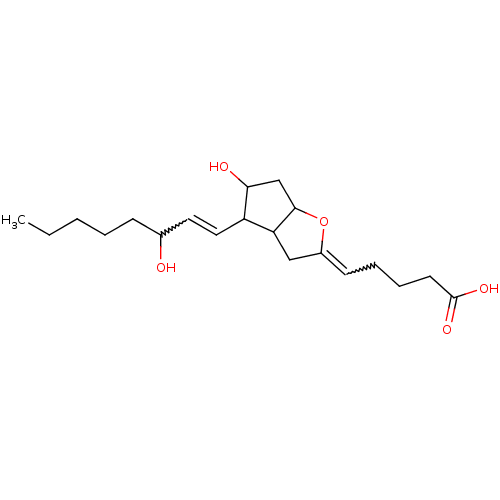
(CAS_35121-78-9 | NSC_5282411 | PGI2)Show SMILES CCCCCC(O)C=CC1C(O)CC2OC(CC12)=CCCCC(O)=O |w:7.6,18.20| Show InChI InChI=1S/C20H32O5/c1-2-3-4-7-14(21)10-11-16-17-12-15(8-5-6-9-20(23)24)25-19(17)13-18(16)22/h8,10-11,14,16-19,21-22H,2-7,9,12-13H2,1H3,(H,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM50101828
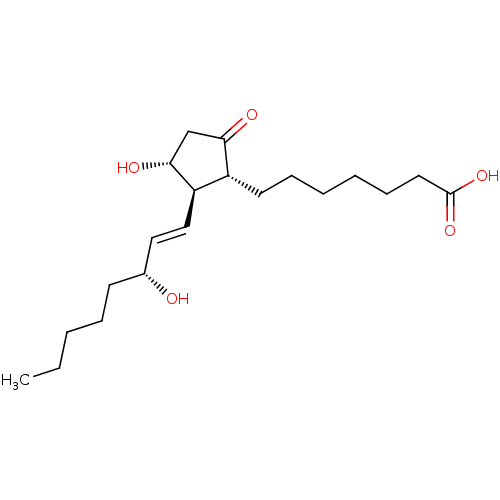
(7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-oct-1...)Show SMILES CCCCC[C@@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16-,17-,19-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM82210

(CAS_54397-85-2 | PG Thromboxane B2 | THROMBOXANE B...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1OC(O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O |r| Show InChI InChI=1S/C20H34O6/c1-2-3-6-9-15(21)12-13-18-16(17(22)14-20(25)26-18)10-7-4-5-8-11-19(23)24/h4,7,12-13,15-18,20-22,25H,2-3,5-6,8-11,14H2,1H3,(H,23,24)/b7-4-,13-12+/t15-,16-,17-,18+,20?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM82212

(CAS_5280881 | NSC_5280881 | PGB2)Show SMILES CCCCCC(O)C=CC1=C(CC=CCCCC(O)=O)C(=O)CC1 |w:7.6,12.11,c:9| Show InChI InChI=1S/C20H30O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h4,7,12,14,17,21H,2-3,5-6,8-11,13,15H2,1H3,(H,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM82211

(6-KETO-PROSTAGLANDIN F1ALPHA | CAS_58962-34-8 | PG...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1CC(=O)CCCCC(O)=O |r| Show InChI InChI=1S/C20H34O6/c1-2-3-4-7-14(21)10-11-16-17(19(24)13-18(16)23)12-15(22)8-5-6-9-20(25)26/h10-11,14,16-19,21,23-24H,2-9,12-13H2,1H3,(H,25,26)/b11-10+/t14-,16+,17+,18+,19-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(RAT) | BDBM34230

((Z)-7-[(1R,2S)-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-ke...)Show SMILES CCCCC[C@H](O)C=C[C@H]1C=CC(=O)[C@@H]1C\C=C/CCCC(O)=O |c:10| Show InChI InChI=1S/C20H30O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h4,7,12-18,21H,2-3,5-6,8-11H2,1H3,(H,23,24)/b7-4-,14-12?/t16-,17-,18+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
J Biol Chem 257: 13570-5 (1982)
BindingDB Entry DOI: 10.7270/Q22F7KXH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6113
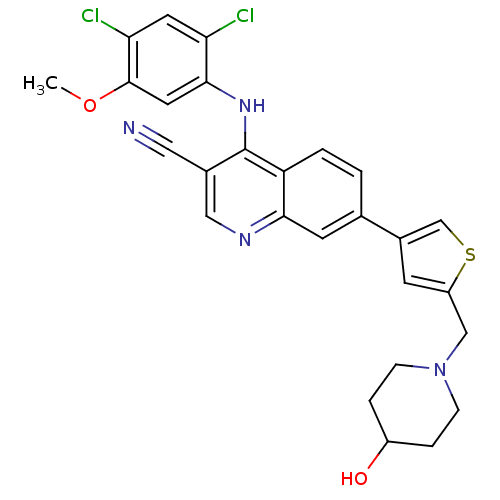
(4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-{5-[(4-h...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2csc(CN3CCC(O)CC3)c2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C27H24Cl2N4O2S/c1-35-26-11-25(22(28)10-23(26)29)32-27-18(12-30)13-31-24-9-16(2-3-21(24)27)17-8-20(36-15-17)14-33-6-4-19(34)5-7-33/h2-3,8-11,13,15,19,34H,4-7,14H2,1H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6115
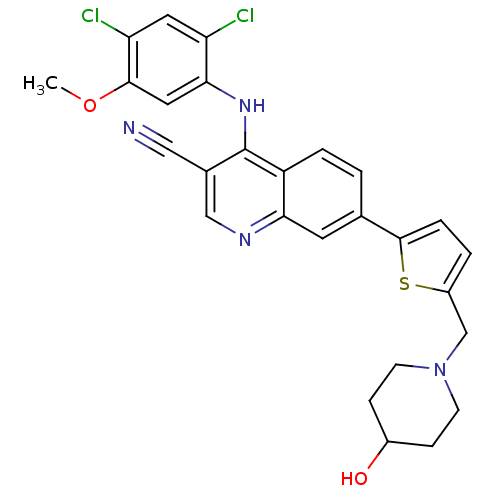
(4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-{5-[(4-h...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2ccc(CN3CCC(O)CC3)s2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C27H24Cl2N4O2S/c1-35-25-12-24(21(28)11-22(25)29)32-27-17(13-30)14-31-23-10-16(2-4-20(23)27)26-5-3-19(36-26)15-33-8-6-18(34)7-9-33/h2-5,10-12,14,18,34H,6-9,15H2,1H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6108
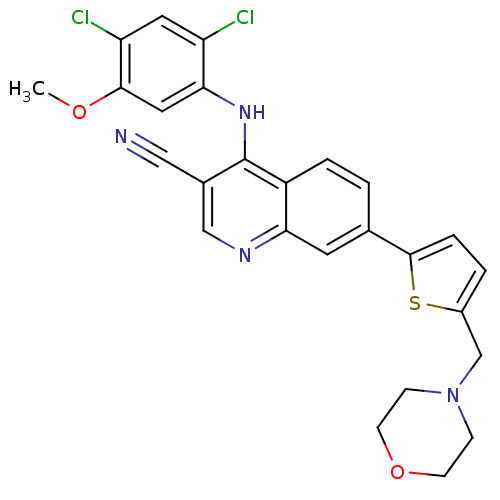
(4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[5-(morp...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2ccc(CN3CCOCC3)s2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H22Cl2N4O2S/c1-33-24-12-23(20(27)11-21(24)28)31-26-17(13-29)14-30-22-10-16(2-4-19(22)26)25-5-3-18(35-25)15-32-6-8-34-9-7-32/h2-5,10-12,14H,6-9,15H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
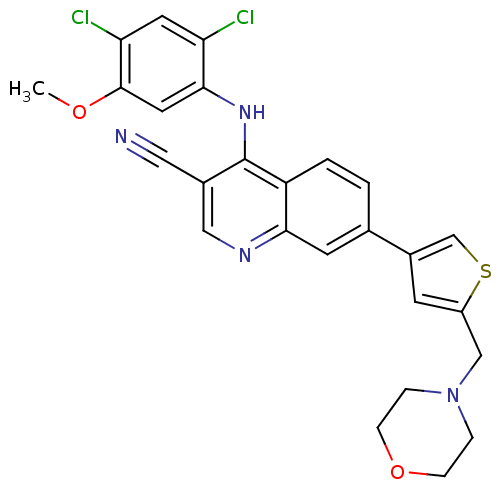
(Homo sapiens (Human)) | BDBM6107
(4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[5-(morp...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2csc(CN3CCOCC3)c2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H22Cl2N4O2S/c1-33-25-11-24(21(27)10-22(25)28)31-26-18(12-29)13-30-23-9-16(2-3-20(23)26)17-8-19(35-15-17)14-32-4-6-34-7-5-32/h2-3,8-11,13,15H,4-7,14H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6114

(3-quinolinecarbonitrile analog 2b | 4-[(2,4-dichlo...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2ccc(CN3CCN(C)CC3)s2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C27H25Cl2N5OS/c1-33-7-9-34(10-8-33)16-19-4-6-26(36-19)17-3-5-20-23(11-17)31-15-18(14-30)27(20)32-24-13-25(35-2)22(29)12-21(24)28/h3-6,11-13,15H,7-10,16H2,1-2H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6112

(3-quinolinecarbonitrile analog 2a | 4-[(2,4-dichlo...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2csc(CN3CCN(C)CC3)c2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C27H25Cl2N5OS/c1-33-5-7-34(8-6-33)15-20-9-18(16-36-20)17-3-4-21-24(10-17)31-14-19(13-30)27(21)32-25-12-26(35-2)23(29)11-22(25)28/h3-4,9-12,14,16H,5-8,15H2,1-2H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6116

(4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[5-(pipe...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2ccc(CN3CCCCC3)s2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C27H24Cl2N4OS/c1-34-25-13-24(21(28)12-22(25)29)32-27-18(14-30)15-31-23-11-17(5-7-20(23)27)26-8-6-19(35-26)16-33-9-3-2-4-10-33/h5-8,11-13,15H,2-4,9-10,16H2,1H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6117

(4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[5-(thio...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2ccc(CN3CCSCC3)s2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H22Cl2N4OS2/c1-33-24-12-23(20(27)11-21(24)28)31-26-17(13-29)14-30-22-10-16(2-4-19(22)26)25-5-3-18(35-25)15-32-6-8-34-9-7-32/h2-5,10-12,14H,6-9,15H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6109

(4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[4-(morp...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2cc(CN3CCOCC3)cs2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H22Cl2N4O2S/c1-33-24-11-23(20(27)10-21(24)28)31-26-18(12-29)13-30-22-9-17(2-3-19(22)26)25-8-16(15-35-25)14-32-4-6-34-7-5-32/h2-3,8-11,13,15H,4-7,14H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
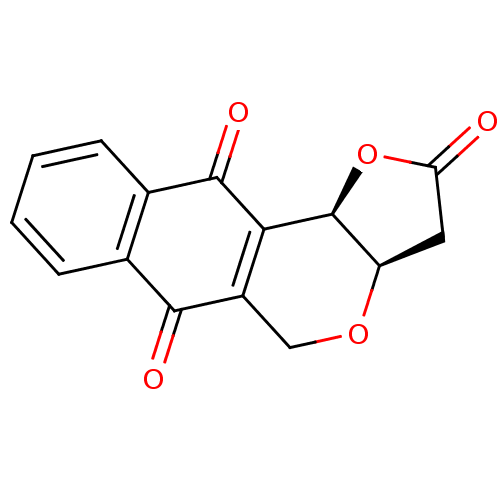
(Homo sapiens (Human)) | BDBM29511
(Pyranonaphthoquinone (PNQ) lactone, 11a)Show SMILES O=C1C[C@H]2OCC3=C([C@H]2O1)C(=O)c1ccccc1C3=O |r,c:6| Show InChI InChI=1S/C15H10O5/c16-11-5-10-15(20-11)12-9(6-19-10)13(17)7-3-1-2-4-8(7)14(12)18/h1-4,10,15H,5-6H2/t10-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
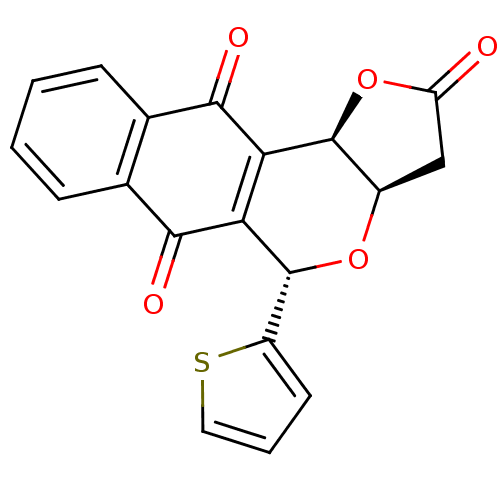
(Homo sapiens (Human)) | BDBM29512
(Pyranonaphthoquinone (PNQ) lactone, 11b)Show SMILES O=C1C[C@H]2O[C@H](c3cccs3)C3=C([C@H]2O1)C(=O)c1ccccc1C3=O |r,c:12| Show InChI InChI=1S/C19H12O5S/c20-13-8-11-18(24-13)14-15(19(23-11)12-6-3-7-25-12)17(22)10-5-2-1-4-9(10)16(14)21/h1-7,11,18-19H,8H2/t11-,18+,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29513
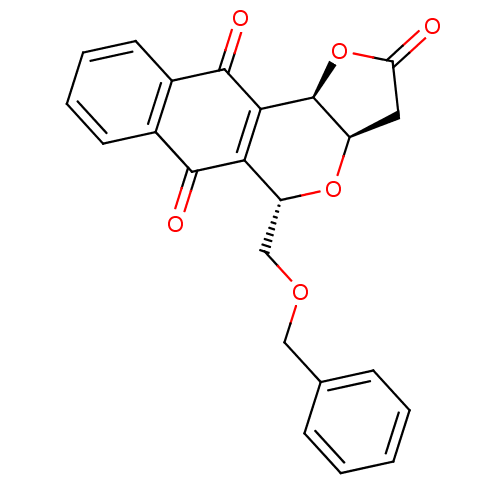
(Pyranonaphthoquinone (PNQ) lactone, 11c)Show SMILES O=C1C[C@H]2O[C@H](COCc3ccccc3)C3=C([C@H]2O1)C(=O)c1ccccc1C3=O |r,c:16| Show InChI InChI=1S/C23H18O6/c24-18-10-16-23(29-18)20-19(21(25)14-8-4-5-9-15(14)22(20)26)17(28-16)12-27-11-13-6-2-1-3-7-13/h1-9,16-17,23H,10-12H2/t16-,17-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29514
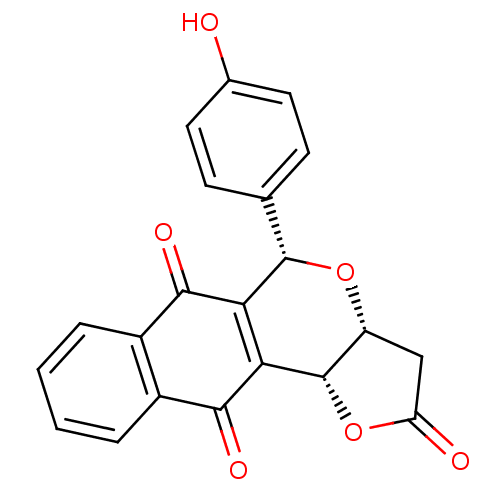
(Pyranonaphthoquinone (PNQ) lactone, 11d)Show SMILES Oc1ccc(cc1)[C@@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1ccccc1C2=O |r,c:17| Show InChI InChI=1S/C21H14O6/c22-11-7-5-10(6-8-11)20-16-17(21-14(26-20)9-15(23)27-21)19(25)13-4-2-1-3-12(13)18(16)24/h1-8,14,20-22H,9H2/t14-,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29515
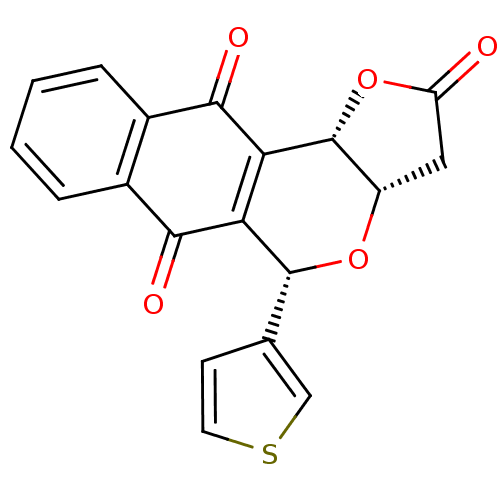
(Pyranonaphthoquinone (PNQ) lactone, 11e)Show SMILES O=C1C[C@@H]2O[C@H](c3ccsc3)C3=C([C@@H]2O1)C(=O)c1ccccc1C3=O |r,c:12| Show InChI InChI=1S/C19H12O5S/c20-13-7-12-19(24-13)15-14(18(23-12)9-5-6-25-8-9)16(21)10-3-1-2-4-11(10)17(15)22/h1-6,8,12,18-19H,7H2/t12-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29516
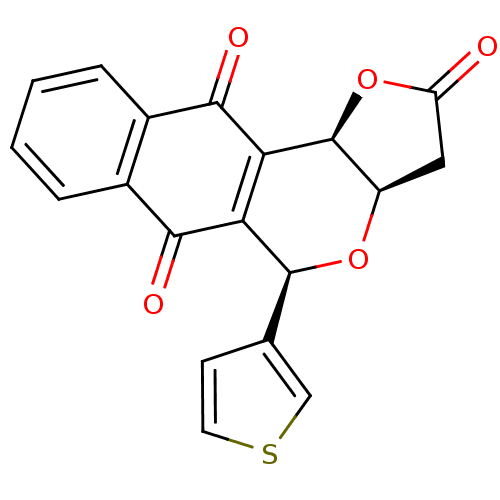
(Pyranonaphthoquinone (PNQ) lactone, 11f)Show SMILES O=C1C[C@H]2O[C@@H](c3ccsc3)C3=C([C@H]2O1)C(=O)c1ccccc1C3=O |r,c:12| Show InChI InChI=1S/C19H12O5S/c20-13-7-12-19(24-13)15-14(18(23-12)9-5-6-25-8-9)16(21)10-3-1-2-4-11(10)17(15)22/h1-6,8,12,18-19H,7H2/t12-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29506
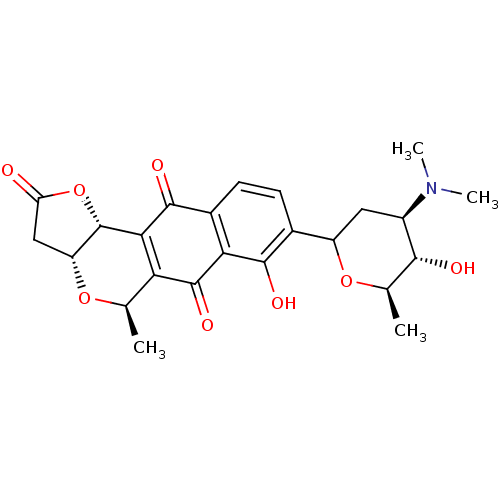
(Lactoquinomycin | Lactoquinomycin A)Show SMILES C[C@H]1OC(C[C@H]([C@@H]1O)N(C)C)c1ccc2C(=O)C3=C([C@@H](C)O[C@@H]4CC(=O)O[C@H]34)C(=O)c2c1O |r,t:18| Show InChI InChI=1S/C24H27NO8/c1-9-17-19(24-15(31-9)8-16(26)33-24)22(29)12-6-5-11(21(28)18(12)23(17)30)14-7-13(25(3)4)20(27)10(2)32-14/h5-6,9-10,13-15,20,24,27-28H,7-8H2,1-4H3/t9-,10-,13-,14?,15-,20-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29517
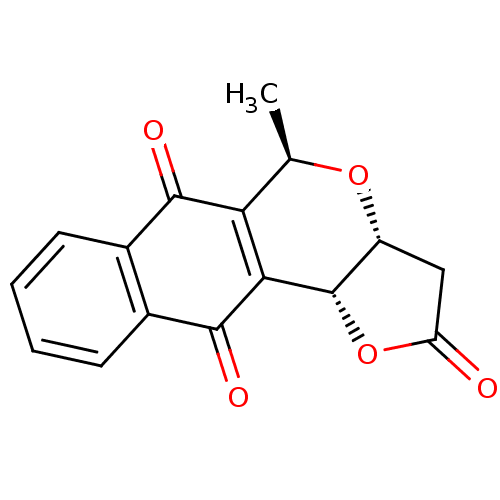
(Pyranonaphthoquinone (PNQ) lactone, 11g)Show SMILES C[C@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1ccccc1C2=O |r,c:10| Show InChI InChI=1S/C16H12O5/c1-7-12-13(16-10(20-7)6-11(17)21-16)15(19)9-5-3-2-4-8(9)14(12)18/h2-5,7,10,16H,6H2,1H3/t7-,10-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29518
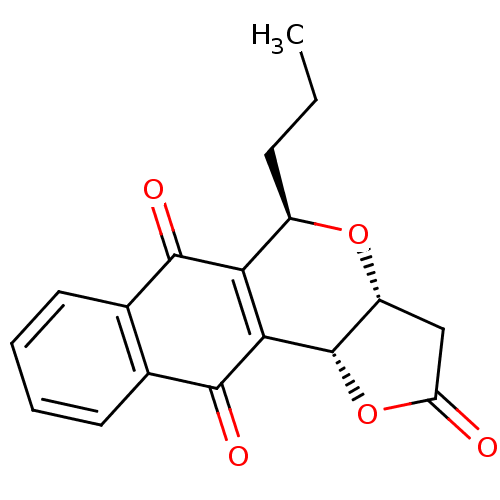
(Pyranonaphthoquinone (PNQ) lactone, 11h)Show SMILES CCC[C@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1ccccc1C2=O |r,c:12| Show InChI InChI=1S/C18H16O5/c1-2-5-11-14-15(18-12(22-11)8-13(19)23-18)17(21)10-7-4-3-6-9(10)16(14)20/h3-4,6-7,11-12,18H,2,5,8H2,1H3/t11-,12-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29508
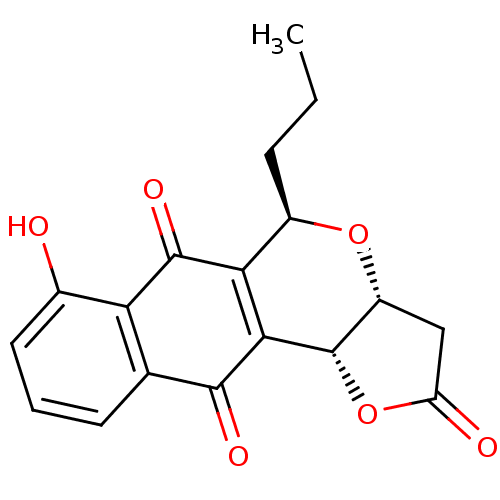
(Frenolicin B)Show SMILES CCC[C@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1c(O)cccc1C2=O |r,c:12| Show InChI InChI=1S/C18H16O6/c1-2-4-10-14-15(18-11(23-10)7-12(20)24-18)16(21)8-5-3-6-9(19)13(8)17(14)22/h3,5-6,10-11,18-19H,2,4,7H2,1H3/t10-,11-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6111

(4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[4-(morp...)Show SMILES COc1cc(Nc2c(cnc3cc(ccc23)-c2cscc2CN2CCOCC2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H22Cl2N4O2S/c1-33-25-10-24(21(27)9-22(25)28)31-26-17(11-29)12-30-23-8-16(2-3-19(23)26)20-15-35-14-18(20)13-32-4-6-34-7-5-32/h2-3,8-10,12,14-15H,4-7,13H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6118
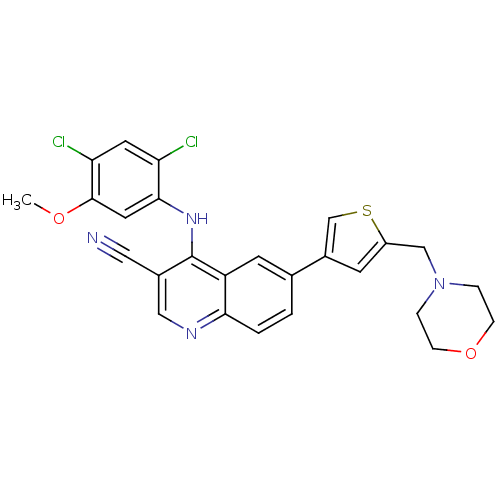
(4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-[5-(morp...)Show SMILES COc1cc(Nc2c(cnc3ccc(cc23)-c2csc(CN3CCOCC3)c2)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H22Cl2N4O2S/c1-33-25-11-24(21(27)10-22(25)28)31-26-18(12-29)13-30-23-3-2-16(9-20(23)26)17-8-19(35-15-17)14-32-4-6-34-7-5-32/h2-3,8-11,13,15H,4-7,14H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 12: 2011-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00302-5
BindingDB Entry DOI: 10.7270/Q2PC30KC |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29519

(Pyranonaphthoquinone (PNQ) lactone, 11i)Show SMILES O=C1C[C@H]2O[C@]3(CCCC3)C3=C([C@H]2O1)C(=O)c1ccccc1C3=O |r,c:11| Show InChI InChI=1S/C19H16O5/c20-13-9-12-18(23-13)14-15(19(24-12)7-3-4-8-19)17(22)11-6-2-1-5-10(11)16(14)21/h1-2,5-6,12,18H,3-4,7-9H2/t12-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 295 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29507

(Kalafungin | Kalamycin | cid_283138)Show SMILES C[C@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1c(O)cccc1C2=O |r,c:10| Show InChI InChI=1S/C16H12O6/c1-6-11-13(16-9(21-6)5-10(18)22-16)14(19)7-3-2-4-8(17)12(7)15(11)20/h2-4,6,9,16-17H,5H2,1H3/t6-,9-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 313 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29520

(Pyranonaphthoquinone (PNQ) lactone, 11j)Show SMILES Oc1cccc(c1)[C@@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1ccccc1C2=O |r,c:17| Show InChI InChI=1S/C21H14O6/c22-11-5-3-4-10(8-11)20-16-17(21-14(26-20)9-15(23)27-21)19(25)13-7-2-1-6-12(13)18(16)24/h1-8,14,20-22H,9H2/t14-,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data