 Found 817 hits with Last Name = 'turnbull' and Initial = 'ap'
Found 817 hits with Last Name = 'turnbull' and Initial = 'ap' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50396691
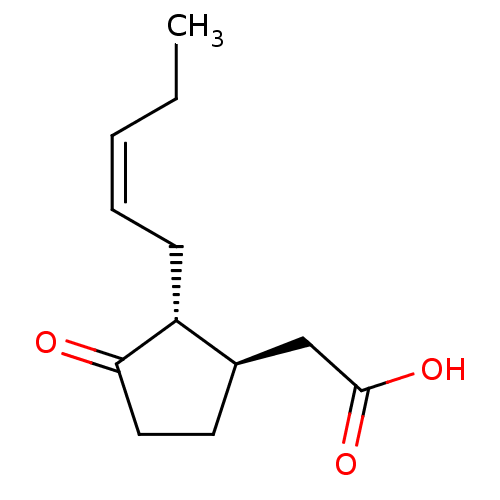
(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C2 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C4
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C4 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C1 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C3 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50446016

(CHEMBL3103330)Show InChI InChI=1S/C15H19Cl2N3O2/c16-12-1-2-14(13(17)11-12)18-3-5-19(6-4-18)15(21)20-7-9-22-10-8-20/h1-2,11H,3-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs |
Bioorg Med Chem 22: 967-77 (2014)
Article DOI: 10.1016/j.bmc.2013.12.050
BindingDB Entry DOI: 10.7270/Q25H7HQM |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082118
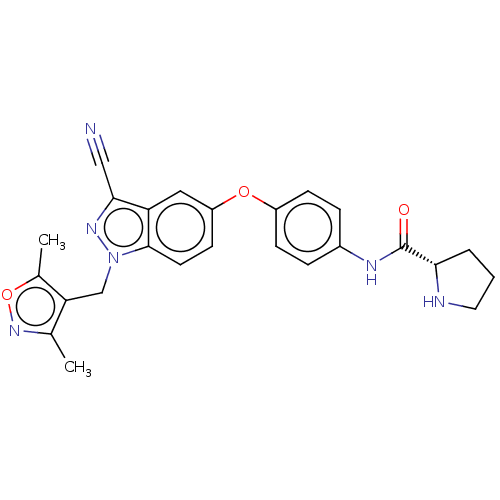
(CHEMBL3422678)Show SMILES Cc1noc(C)c1Cn1nc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C25H24N6O3/c1-15-21(16(2)34-30-15)14-31-24-10-9-19(12-20(24)23(13-26)29-31)33-18-7-5-17(6-8-18)28-25(32)22-4-3-11-27-22/h5-10,12,22,27H,3-4,11,14H2,1-2H3,(H,28,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082117
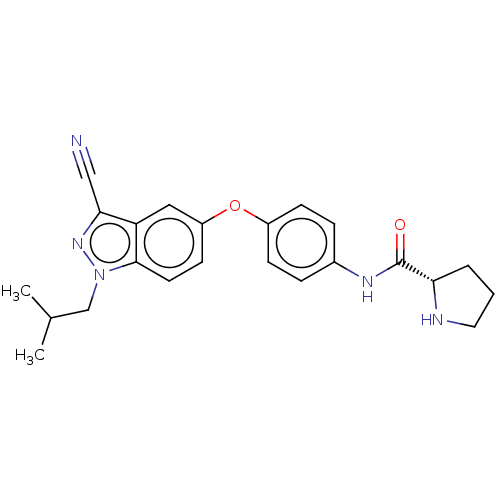
(CHEMBL3422677)Show SMILES CC(C)Cn1nc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C23H25N5O2/c1-15(2)14-28-22-10-9-18(12-19(22)21(13-24)27-28)30-17-7-5-16(6-8-17)26-23(29)20-4-3-11-25-20/h5-10,12,15,20,25H,3-4,11,14H2,1-2H3,(H,26,29)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591272
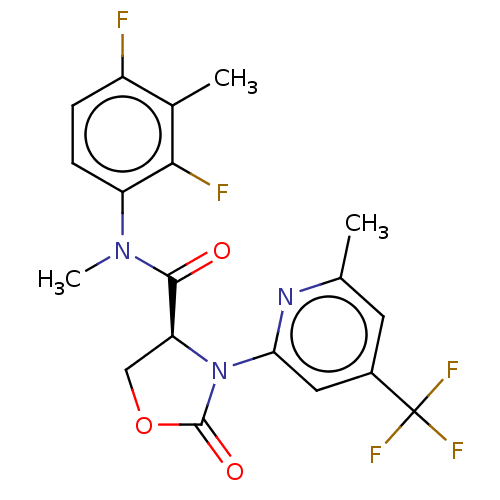
(CHEMBL5208956)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(C)c1F |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591280

(CHEMBL5205456)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(Cl)c1F |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591283
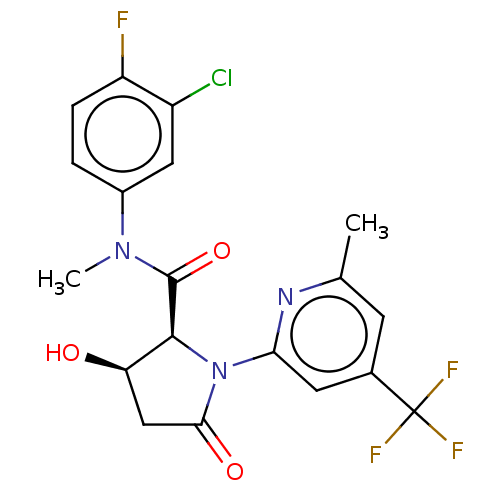
(CHEMBL5190089)Show SMILES CN(C(=O)[C@@H]1[C@H](O)CC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(Cl)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396653

(CHEMBL2172122)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2cc(Br)ccc2C1 Show InChI InChI=1S/C16H14BrNO4S/c17-14-5-4-13-10-18(7-6-11(13)8-14)23(21,22)15-3-1-2-12(9-15)16(19)20/h1-5,8-9H,6-7,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591246
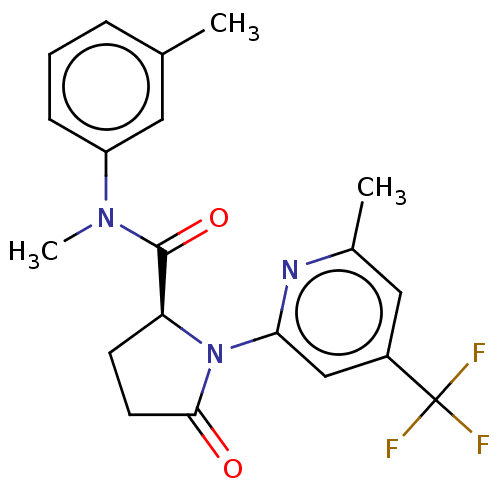
(CHEMBL5175531)Show SMILES CN(C(=O)[C@@H]1CCC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591270
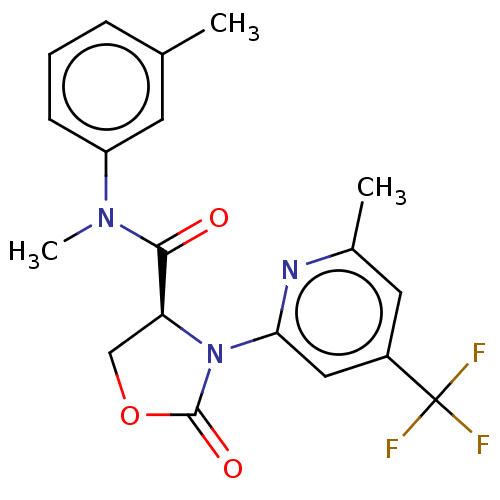
(CHEMBL5176919)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591285
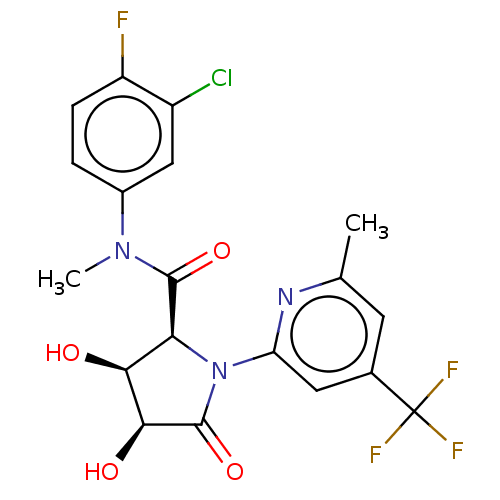
(CHEMBL5191330)Show SMILES CN(C(=O)[C@@H]1[C@H](O)[C@H](O)C(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(Cl)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591248

(CHEMBL5206992)Show SMILES CN(C(=O)[C@@H]1[C@H](O)CCN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082114
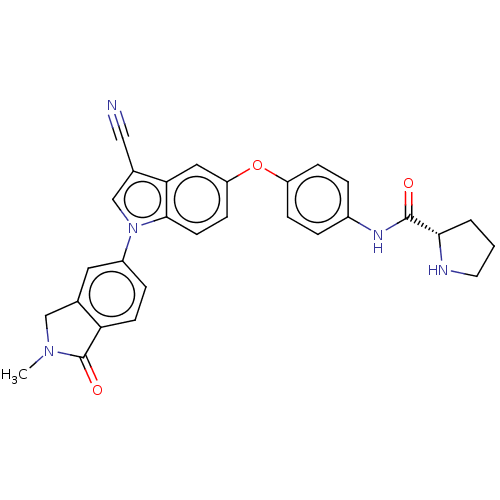
(CHEMBL3422674)Show SMILES CN1Cc2cc(ccc2C1=O)-n1cc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C29H25N5O3/c1-33-16-18-13-21(6-10-24(18)29(33)36)34-17-19(15-30)25-14-23(9-11-27(25)34)37-22-7-4-20(5-8-22)32-28(35)26-3-2-12-31-26/h4-11,13-14,17,26,31H,2-3,12,16H2,1H3,(H,32,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396661
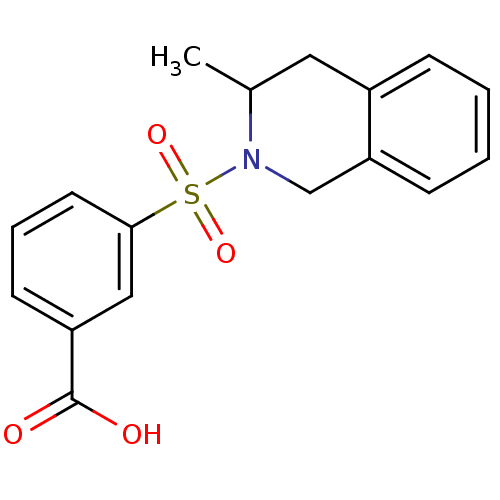
(CHEMBL2172110)Show InChI InChI=1S/C17H17NO4S/c1-12-9-13-5-2-3-6-15(13)11-18(12)23(21,22)16-8-4-7-14(10-16)17(19)20/h2-8,10,12H,9,11H2,1H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396695

(CHEMBL2172121)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2cc(Cl)ccc2C1 Show InChI InChI=1S/C16H14ClNO4S/c17-14-5-4-13-10-18(7-6-11(13)8-14)23(21,22)15-3-1-2-12(9-15)16(19)20/h1-5,8-9H,6-7,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591255

(CHEMBL5180095)Show SMILES CC(C)N(C(=O)[C@@H]1CCCN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396694

(CHEMBL2172112)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2c(C1)cccc2[N+]([O-])=O Show InChI InChI=1S/C16H14N2O6S/c19-16(20)11-3-1-5-13(9-11)25(23,24)17-8-7-14-12(10-17)4-2-6-15(14)18(21)22/h1-6,9H,7-8,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591271

(CHEMBL5200410)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396693
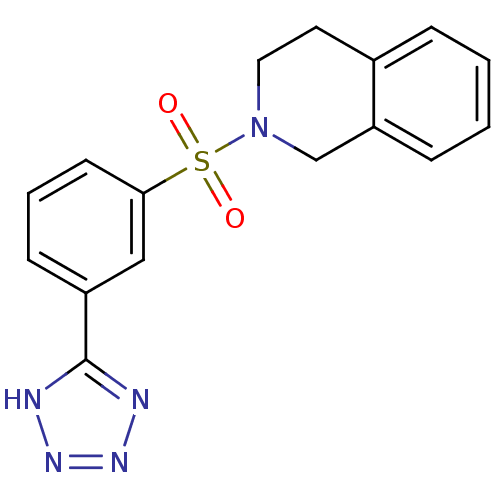
(CHEMBL2172067)Show SMILES O=S(=O)(N1CCc2ccccc2C1)c1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C16H15N5O2S/c22-24(23,21-9-8-12-4-1-2-5-14(12)11-21)15-7-3-6-13(10-15)16-17-19-20-18-16/h1-7,10H,8-9,11H2,(H,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50446017
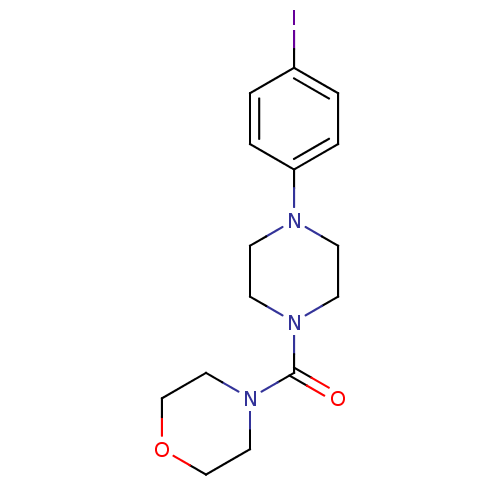
(CHEMBL3103349)Show InChI InChI=1S/C15H20IN3O2/c16-13-1-3-14(4-2-13)17-5-7-18(8-6-17)15(20)19-9-11-21-12-10-19/h1-4H,5-12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs |
Bioorg Med Chem 22: 967-77 (2014)
Article DOI: 10.1016/j.bmc.2013.12.050
BindingDB Entry DOI: 10.7270/Q25H7HQM |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396658

(CHEMBL2172114)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2c(Br)cccc2C1 Show InChI InChI=1S/C16H14BrNO4S/c17-15-6-2-4-12-10-18(8-7-14(12)15)23(21,22)13-5-1-3-11(9-13)16(19)20/h1-6,9H,7-8,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50430719
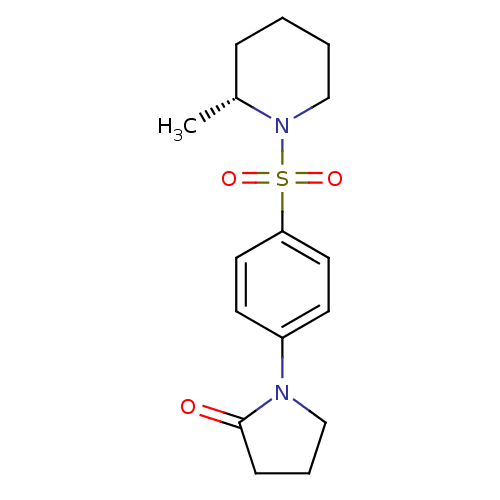
(CHEMBL2333522)Show SMILES C[C@@H]1CCCCN1S(=O)(=O)c1ccc(cc1)N1CCCC1=O |r| Show InChI InChI=1S/C16H22N2O3S/c1-13-5-2-3-12-18(13)22(20,21)15-9-7-14(8-10-15)17-11-4-6-16(17)19/h7-10,13H,2-6,11-12H2,1H3/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) overexpressed in human HCT116 cells assessed as inhibition of aerobic reduction of dinitrobenzamide... |
Eur J Med Chem 62: 738-44 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.047
BindingDB Entry DOI: 10.7270/Q2H133CX |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082116
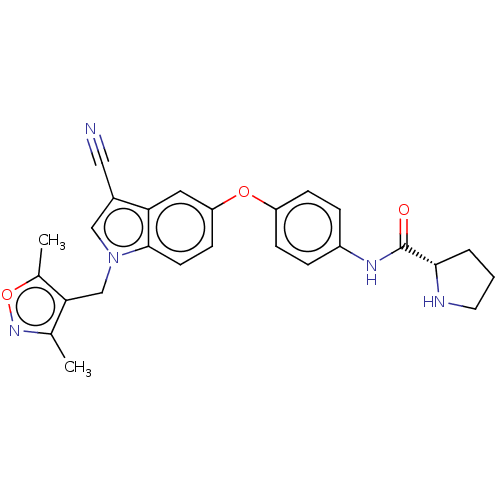
(CHEMBL3422676)Show SMILES Cc1noc(C)c1Cn1cc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C26H25N5O3/c1-16-23(17(2)34-30-16)15-31-14-18(13-27)22-12-21(9-10-25(22)31)33-20-7-5-19(6-8-20)29-26(32)24-4-3-11-28-24/h5-10,12,14,24,28H,3-4,11,15H2,1-2H3,(H,29,32)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396659
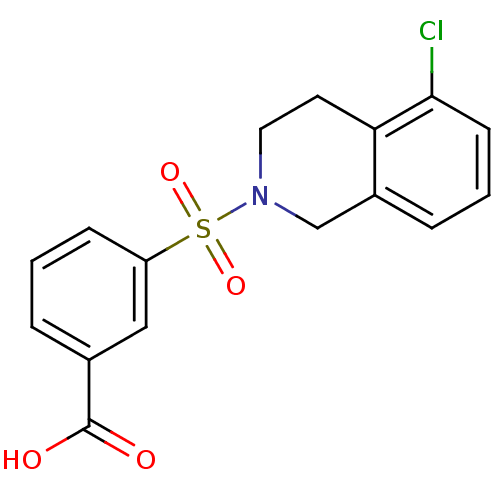
(CHEMBL2172113)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2c(Cl)cccc2C1 Show InChI InChI=1S/C16H14ClNO4S/c17-15-6-2-4-12-10-18(8-7-14(12)15)23(21,22)13-5-1-3-11(9-13)16(19)20/h1-6,9H,7-8,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396653

(CHEMBL2172122)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2cc(Br)ccc2C1 Show InChI InChI=1S/C16H14BrNO4S/c17-14-5-4-13-10-18(7-6-11(13)8-14)23(21,22)15-3-1-2-12(9-15)16(19)20/h1-5,8-9H,6-7,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50446018
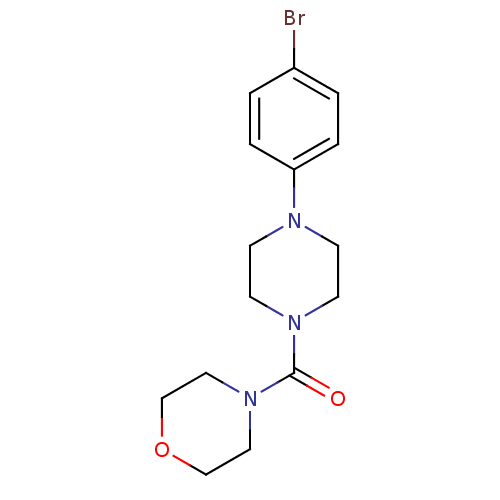
(CHEMBL3103348)Show InChI InChI=1S/C15H20BrN3O2/c16-13-1-3-14(4-2-13)17-5-7-18(8-6-17)15(20)19-9-11-21-12-10-19/h1-4H,5-12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs |
Bioorg Med Chem 22: 967-77 (2014)
Article DOI: 10.1016/j.bmc.2013.12.050
BindingDB Entry DOI: 10.7270/Q25H7HQM |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396692

(CHEMBL2172087)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2ccc(Br)cc2C1 Show InChI InChI=1S/C16H14BrNO4S/c17-14-5-4-11-6-7-18(10-13(11)8-14)23(21,22)15-3-1-2-12(9-15)16(19)20/h1-5,8-9H,6-7,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396704
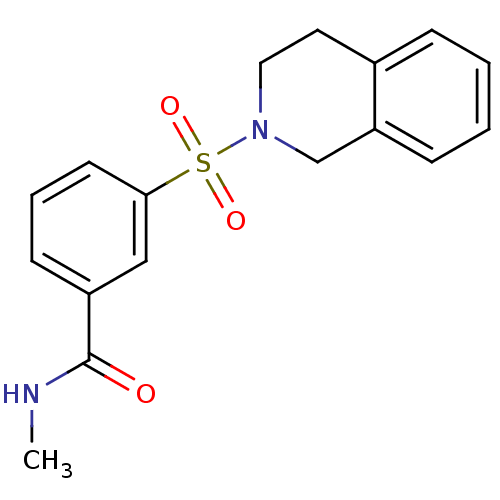
(CHEMBL2172072)Show InChI InChI=1S/C17H18N2O3S/c1-18-17(20)14-7-4-8-16(11-14)23(21,22)19-10-9-13-5-2-3-6-15(13)12-19/h2-8,11H,9-10,12H2,1H3,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396707
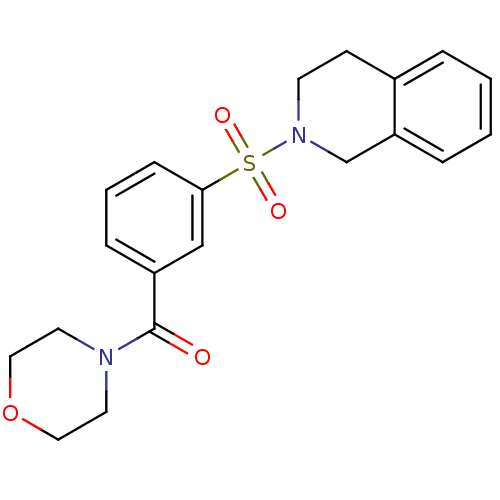
(CHEMBL2172069)Show SMILES O=C(N1CCOCC1)c1cccc(c1)S(=O)(=O)N1CCc2ccccc2C1 Show InChI InChI=1S/C20H22N2O4S/c23-20(21-10-12-26-13-11-21)17-6-3-7-19(14-17)27(24,25)22-9-8-16-4-1-2-5-18(16)15-22/h1-7,14H,8-13,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50446015
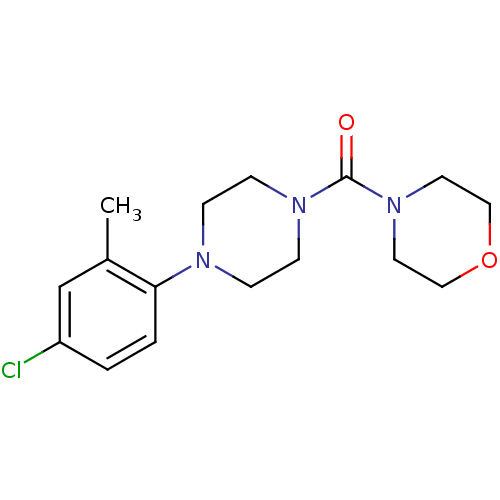
(CHEMBL3103331)Show InChI InChI=1S/C16H22ClN3O2/c1-13-12-14(17)2-3-15(13)18-4-6-19(7-5-18)16(21)20-8-10-22-11-9-20/h2-3,12H,4-11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs |
Bioorg Med Chem 22: 967-77 (2014)
Article DOI: 10.1016/j.bmc.2013.12.050
BindingDB Entry DOI: 10.7270/Q25H7HQM |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396676
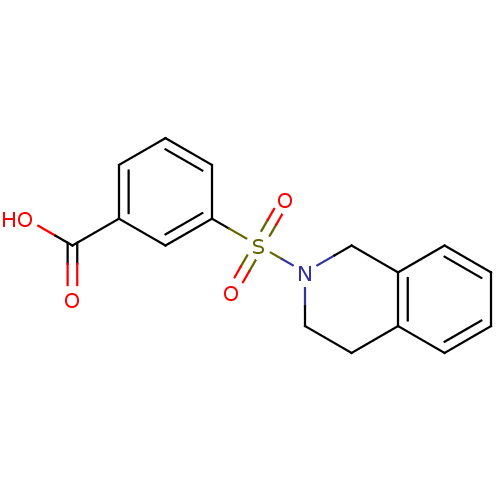
(CHEMBL1566492)Show InChI InChI=1S/C16H15NO4S/c18-16(19)13-6-3-7-15(10-13)22(20,21)17-9-8-12-4-1-2-5-14(12)11-17/h1-7,10H,8-9,11H2,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50446014

(CHEMBL3103332)Show InChI InChI=1S/C16H22ClN3O3/c17-14-1-2-15(13(11-14)12-21)18-3-5-19(6-4-18)16(22)20-7-9-23-10-8-20/h1-2,11,21H,3-10,12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs |
Bioorg Med Chem 22: 967-77 (2014)
Article DOI: 10.1016/j.bmc.2013.12.050
BindingDB Entry DOI: 10.7270/Q25H7HQM |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396650

(CHEMBL2172220)Show InChI InChI=1S/C17H17NO4S/c1-12-5-6-13-7-8-18(11-15(13)9-12)23(21,22)16-4-2-3-14(10-16)17(19)20/h2-6,9-10H,7-8,11H2,1H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396703
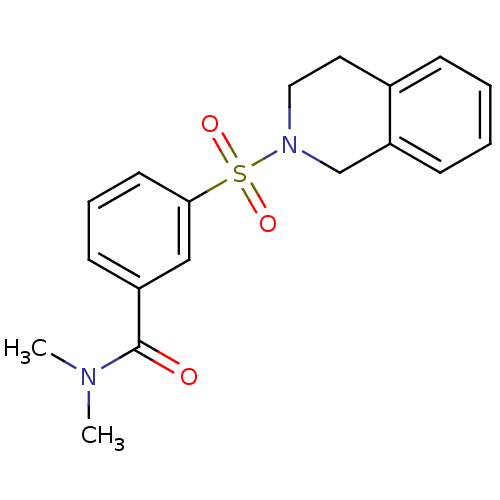
(CHEMBL2172073)Show SMILES CN(C)C(=O)c1cccc(c1)S(=O)(=O)N1CCc2ccccc2C1 Show InChI InChI=1S/C18H20N2O3S/c1-19(2)18(21)15-8-5-9-17(12-15)24(22,23)20-11-10-14-6-3-4-7-16(14)13-20/h3-9,12H,10-11,13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
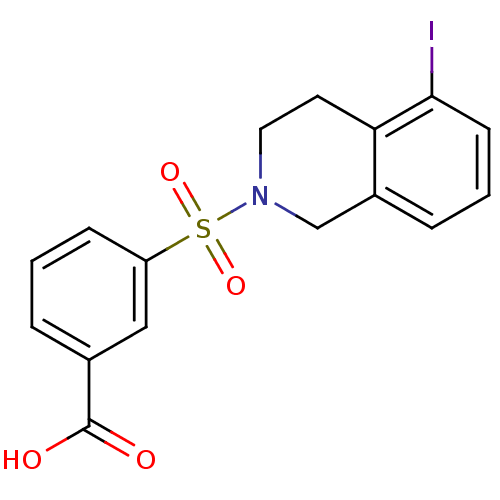
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396657
(CHEMBL2172115)Show InChI InChI=1S/C16H14INO4S/c17-15-6-2-4-12-10-18(8-7-14(12)15)23(21,22)13-5-1-3-11(9-13)16(19)20/h1-6,9H,7-8,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396719

(CHEMBL2172088)Show InChI InChI=1S/C16H14INO4S/c17-14-5-4-11-6-7-18(10-13(11)8-14)23(21,22)15-3-1-2-12(9-15)16(19)20/h1-5,8-9H,6-7,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396658

(CHEMBL2172114)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2c(Br)cccc2C1 Show InChI InChI=1S/C16H14BrNO4S/c17-15-6-2-4-12-10-18(8-7-14(12)15)23(21,22)13-5-1-3-11(9-13)16(19)20/h1-6,9H,7-8,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2
(Homo sapiens (Human)) | BDBM50082117

(CHEMBL3422677)Show SMILES CC(C)Cn1nc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C23H25N5O2/c1-15(2)14-28-22-10-9-18(12-19(22)21(13-24)27-28)30-17-7-5-16(6-8-17)26-23(29)20-4-3-11-25-20/h5-10,12,15,20,25H,3-4,11,14H2,1-2H3,(H,26,29)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB2 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591273

(CHEMBL5205799)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1cccc(Cl)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50430718
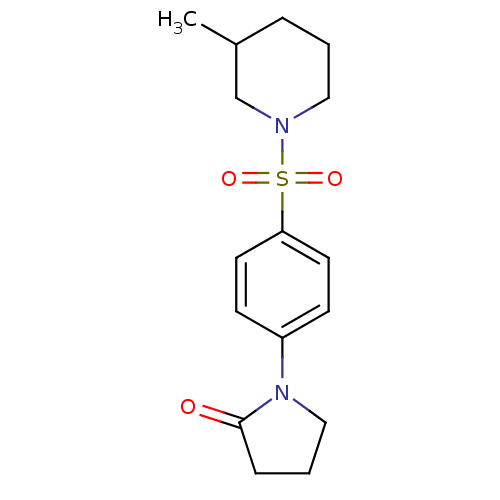
(CHEMBL2333523)Show InChI InChI=1S/C16H22N2O3S/c1-13-4-2-10-17(12-13)22(20,21)15-8-6-14(7-9-15)18-11-3-5-16(18)19/h6-9,13H,2-5,10-12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) overexpressed in human HCT116 cells assessed as inhibition of aerobic reduction of dinitrobenzamide... |
Eur J Med Chem 62: 738-44 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.047
BindingDB Entry DOI: 10.7270/Q2H133CX |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591279
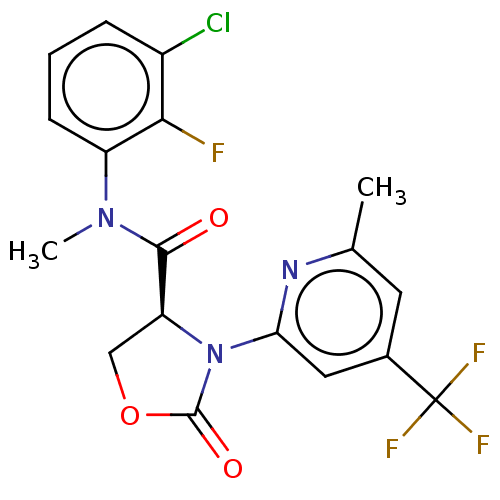
(CHEMBL5191696)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1cccc(Cl)c1F |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591274
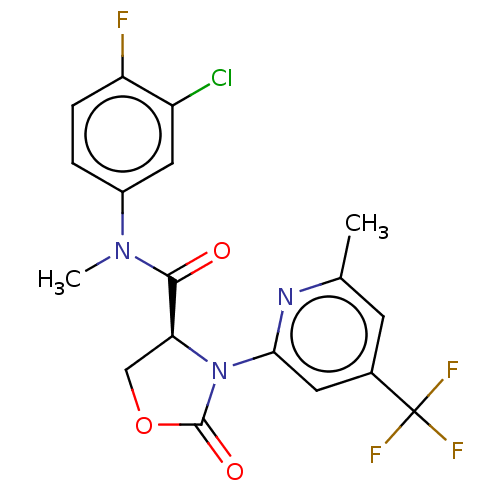
(CHEMBL5184660)Show SMILES CN(C(=O)[C@@H]1COC(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(Cl)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396659

(CHEMBL2172113)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2c(Cl)cccc2C1 Show InChI InChI=1S/C16H14ClNO4S/c17-15-6-2-4-12-10-18(8-7-14(12)15)23(21,22)13-5-1-3-11(9-13)16(19)20/h1-6,9H,7-8,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396722

(CHEMBL2172116)Show InChI InChI=1S/C16H15NO5S/c18-15-6-2-4-12-10-17(8-7-14(12)15)23(21,22)13-5-1-3-11(9-13)16(19)20/h1-6,9,18H,7-8,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396656
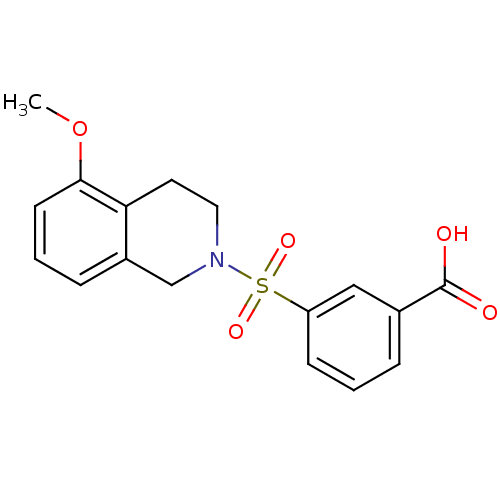
(CHEMBL2172117)Show SMILES COc1cccc2CN(CCc12)S(=O)(=O)c1cccc(c1)C(O)=O Show InChI InChI=1S/C17H17NO5S/c1-23-16-7-3-5-13-11-18(9-8-15(13)16)24(21,22)14-6-2-4-12(10-14)17(19)20/h2-7,10H,8-9,11H2,1H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396655
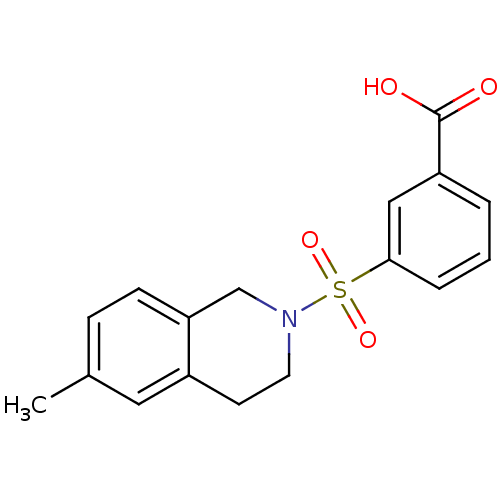
(CHEMBL2172118)Show InChI InChI=1S/C17H17NO4S/c1-12-5-6-15-11-18(8-7-13(15)9-12)23(21,22)16-4-2-3-14(10-16)17(19)20/h2-6,9-10H,7-8,11H2,1H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396646
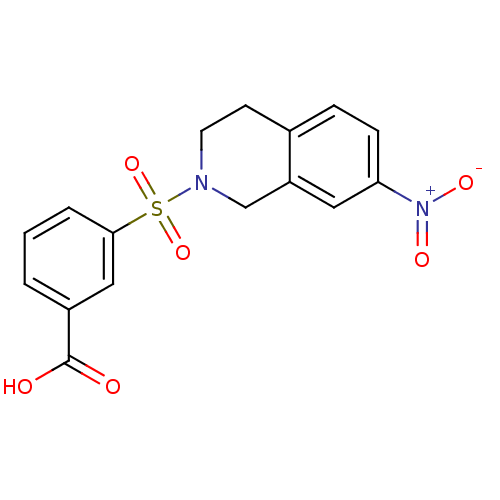
(CHEMBL2172084)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)N1CCc2ccc(cc2C1)[N+]([O-])=O Show InChI InChI=1S/C16H14N2O6S/c19-16(20)12-2-1-3-15(9-12)25(23,24)17-7-6-11-4-5-14(18(21)22)8-13(11)10-17/h1-5,8-9H,6-7,10H2,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data