 Found 1350 hits with Last Name = 'collins' and Initial = 'c'
Found 1350 hits with Last Name = 'collins' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144020
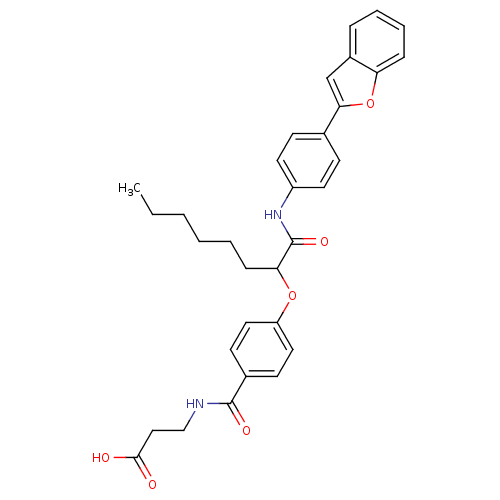
(3-{4-[1-(4-Benzofuran-2-yl-phenylcarbamoyl)-heptyl...)Show SMILES CCCCCCC(Oc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C32H34N2O6/c1-2-3-4-5-10-28(39-26-17-13-23(14-18-26)31(37)33-20-19-30(35)36)32(38)34-25-15-11-22(12-16-25)29-21-24-8-6-7-9-27(24)40-29/h6-9,11-18,21,28H,2-5,10,19-20H2,1H3,(H,33,37)(H,34,38)(H,35,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144008

(3-{4-[2-(4-Benzofuran-2-yl-phenylcarbamoyl)-2-(4-t...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C37H36N2O5/c1-37(2,3)29-16-12-25(13-17-29)31(22-24-8-10-27(11-9-24)35(42)38-21-20-34(40)41)36(43)39-30-18-14-26(15-19-30)33-23-28-6-4-5-7-32(28)44-33/h4-19,23,31H,20-22H2,1-3H3,(H,38,42)(H,39,43)(H,40,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144001

(3-{4-[1-(2',4'-Dichloro-biphenyl-4-ylcarbamoyl)-he...)Show SMILES CCCCCCC(Oc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C30H32Cl2N2O5/c1-2-3-4-5-6-27(39-24-14-9-21(10-15-24)29(37)33-18-17-28(35)36)30(38)34-23-12-7-20(8-13-23)25-16-11-22(31)19-26(25)32/h7-16,19,27H,2-6,17-18H2,1H3,(H,33,37)(H,34,38)(H,35,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144015
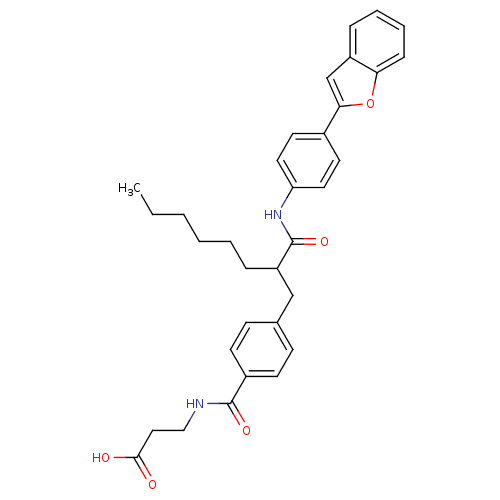
(3-{4-[2-(4-Benzofuran-2-yl-phenylcarbamoyl)-octyl]...)Show SMILES CCCCCCC(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C33H36N2O5/c1-2-3-4-5-9-27(21-23-11-13-25(14-12-23)32(38)34-20-19-31(36)37)33(39)35-28-17-15-24(16-18-28)30-22-26-8-6-7-10-29(26)40-30/h6-8,10-18,22,27H,2-5,9,19-21H2,1H3,(H,34,38)(H,35,39)(H,36,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144002

(3-{4-[2-(4-tert-Butyl-phenyl)-2-(2',4'-dichloro-bi...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C35H34Cl2N2O4/c1-35(2,3)26-12-8-24(9-13-26)30(20-22-4-6-25(7-5-22)33(42)38-19-18-32(40)41)34(43)39-28-15-10-23(11-16-28)29-17-14-27(36)21-31(29)37/h4-17,21,30H,18-20H2,1-3H3,(H,38,42)(H,39,43)(H,40,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144016

(3-{4-[(Biphenyl-4-ylcarbamoyl)-(4-tert-butyl-pheny...)Show SMILES CC(C)(C)c1ccc(cc1)C(Oc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C34H34N2O5/c1-34(2,3)27-15-9-25(10-16-27)31(41-29-19-13-26(14-20-29)32(39)35-22-21-30(37)38)33(40)36-28-17-11-24(12-18-28)23-7-5-4-6-8-23/h4-20,31H,21-22H2,1-3H3,(H,35,39)(H,36,40)(H,37,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144004

(3-{4-[2-(Biphenyl-4-ylcarbamoyl)-2-(4-tert-butyl-p...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C35H36N2O4/c1-35(2,3)29-17-13-27(14-18-29)31(23-24-9-11-28(12-10-24)33(40)36-22-21-32(38)39)34(41)37-30-19-15-26(16-20-30)25-7-5-4-6-8-25/h4-20,31H,21-23H2,1-3H3,(H,36,40)(H,37,41)(H,38,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144006
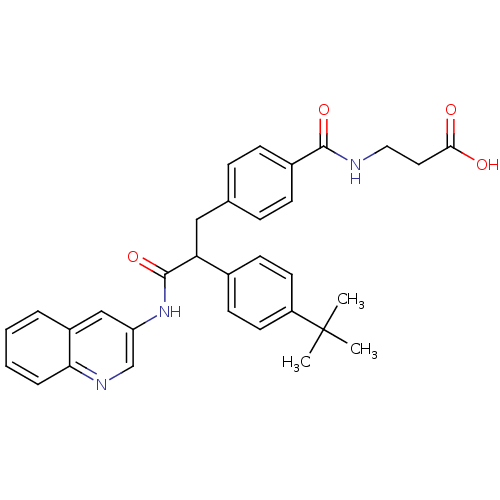
(3-{4-[2-(4-tert-Butyl-phenyl)-2-(quinolin-3-ylcarb...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1cnc2ccccc2c1 Show InChI InChI=1S/C32H33N3O4/c1-32(2,3)25-14-12-22(13-15-25)27(31(39)35-26-19-24-6-4-5-7-28(24)34-20-26)18-21-8-10-23(11-9-21)30(38)33-17-16-29(36)37/h4-15,19-20,27H,16-18H2,1-3H3,(H,33,38)(H,35,39)(H,36,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 11.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 1/2/3/4/5/6/7/8/9
(Homo sapiens (Human)) | BDBM50144001

(3-{4-[1-(2',4'-Dichloro-biphenyl-4-ylcarbamoyl)-he...)Show SMILES CCCCCCC(Oc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C30H32Cl2N2O5/c1-2-3-4-5-6-27(39-24-14-9-21(10-15-24)29(37)33-18-17-28(35)36)30(38)34-23-12-7-20(8-13-23)25-16-11-22(31)19-26(25)32/h7-16,19,27H,2-6,17-18H2,1H3,(H,33,37)(H,34,38)(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against glucagon induced human adenylate cyclase |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 1/2/3/4/5/6/7/8/9
(Homo sapiens (Human)) | BDBM50144020

(3-{4-[1-(4-Benzofuran-2-yl-phenylcarbamoyl)-heptyl...)Show SMILES CCCCCCC(Oc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C32H34N2O6/c1-2-3-4-5-10-28(39-26-17-13-23(14-18-26)31(37)33-20-19-30(35)36)32(38)34-25-15-11-22(12-16-25)29-21-24-8-6-7-9-27(24)40-29/h6-9,11-18,21,28H,2-5,10,19-20H2,1H3,(H,33,37)(H,34,38)(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against glucagon induced human adenylate cyclase |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 1/2/3/4/5/6/7/8/9
(Homo sapiens (Human)) | BDBM50144015

(3-{4-[2-(4-Benzofuran-2-yl-phenylcarbamoyl)-octyl]...)Show SMILES CCCCCCC(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C33H36N2O5/c1-2-3-4-5-9-27(21-23-11-13-25(14-12-23)32(38)34-20-19-31(36)37)33(39)35-28-17-15-24(16-18-28)30-22-26-8-6-7-10-29(26)40-30/h6-8,10-18,22,27H,2-5,9,19-21H2,1H3,(H,34,38)(H,35,39)(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against glucagon induced human adenylate cyclase |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144011

(3-{4-[2-(4-tert-Butyl-phenyl)-2-(4-ethyl-phenylcar...)Show SMILES CCc1ccc(NC(=O)C(Cc2ccc(cc2)C(=O)NCCC(O)=O)c2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C31H36N2O4/c1-5-21-8-16-26(17-9-21)33-30(37)27(23-12-14-25(15-13-23)31(2,3)4)20-22-6-10-24(11-7-22)29(36)32-19-18-28(34)35/h6-17,27H,5,18-20H2,1-4H3,(H,32,36)(H,33,37)(H,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144019
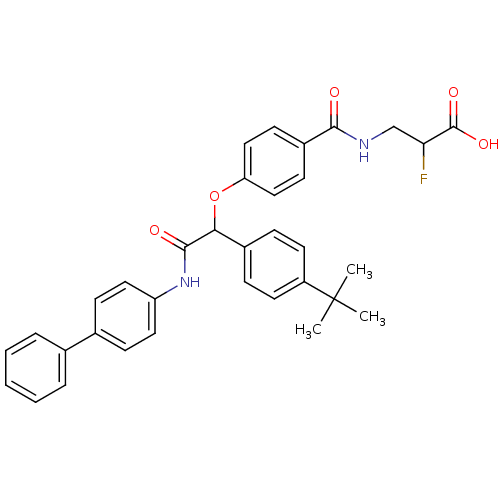
(3-{4-[(Biphenyl-4-ylcarbamoyl)-(4-tert-butyl-pheny...)Show SMILES CC(C)(C)c1ccc(cc1)C(Oc1ccc(cc1)C(=O)NCC(F)C(O)=O)C(=O)Nc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C34H33FN2O5/c1-34(2,3)26-15-9-24(10-16-26)30(32(39)37-27-17-11-23(12-18-27)22-7-5-4-6-8-22)42-28-19-13-25(14-20-28)31(38)36-21-29(35)33(40)41/h4-20,29-30H,21H2,1-3H3,(H,36,38)(H,37,39)(H,40,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50511169

(CHEMBL4551377)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(N)nc(N)c1 |r| Show InChI InChI=1S/C20H26N6O2.2C2HF3O2/c21-15(9-13-5-2-1-3-6-13)20(28)26-8-4-7-16(26)19(27)24-12-14-10-17(22)25-18(23)11-14;2*3-2(4,5)1(6)7/h1-3,5-6,10-11,15-16H,4,7-9,12,21H2,(H,24,27)(H4,22,23,25);2*(H,6,7)/t15-,16+;;/m1../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin using varying levels of Tos-Gly-Pro-Arg-AMC-TFA as substrate by fluorescence based Michaelis-Menten equation analy... |
J Med Chem 63: 3274-3289 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02061
BindingDB Entry DOI: 10.7270/Q2WM1HRB |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 1/2/3/4/5/6/7/8/9
(Homo sapiens (Human)) | BDBM50144008

(3-{4-[2-(4-Benzofuran-2-yl-phenylcarbamoyl)-2-(4-t...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C37H36N2O5/c1-37(2,3)29-16-12-25(13-17-29)31(22-24-8-10-27(11-9-24)35(42)38-21-20-34(40)41)36(43)39-30-18-14-26(15-19-30)33-23-28-6-4-5-7-32(28)44-33/h4-19,23,31H,20-22H2,1-3H3,(H,38,42)(H,39,43)(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against glucagon induced human adenylate cyclase |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144005

(3-{4-[2-(4-tert-Butyl-phenyl)-2-(4-hydroxymethyl-p...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(CO)cc1 Show InChI InChI=1S/C30H34N2O5/c1-30(2,3)24-12-10-22(11-13-24)26(29(37)32-25-14-6-21(19-33)7-15-25)18-20-4-8-23(9-5-20)28(36)31-17-16-27(34)35/h4-15,26,33H,16-19H2,1-3H3,(H,31,36)(H,32,37)(H,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183974
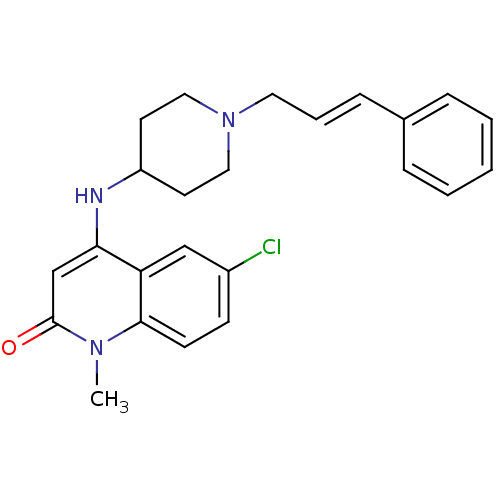
((E)-6-chloro-4-(1-cinnamylpiperidin-4-ylamino)-1-m...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(C\C=C\c3ccccc3)CC2)cc1=O Show InChI InChI=1S/C24H26ClN3O/c1-27-23-10-9-19(25)16-21(23)22(17-24(27)29)26-20-11-14-28(15-12-20)13-5-8-18-6-3-2-4-7-18/h2-10,16-17,20,26H,11-15H2,1H3/b8-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 1/2/3/4/5/6/7/8/9
(Homo sapiens (Human)) | BDBM50144002

(3-{4-[2-(4-tert-Butyl-phenyl)-2-(2',4'-dichloro-bi...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C35H34Cl2N2O4/c1-35(2,3)26-12-8-24(9-13-26)30(20-22-4-6-25(7-5-22)33(42)38-19-18-32(40)41)34(43)39-28-15-10-23(11-16-28)29-17-14-27(36)21-31(29)37/h4-17,21,30H,18-20H2,1-3H3,(H,38,42)(H,39,43)(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against glucagon induced human adenylate cyclase |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144003
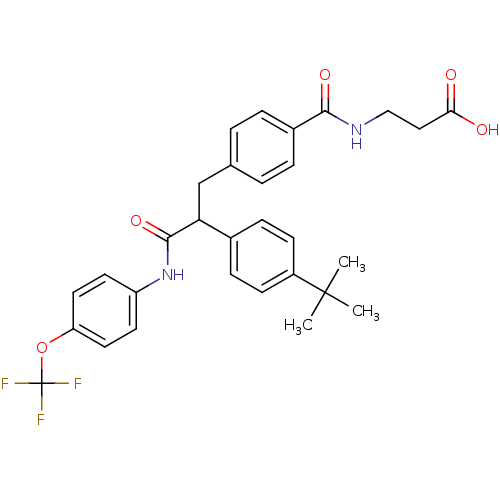
(3-{4-[2-(4-tert-Butyl-phenyl)-2-(4-trifluoromethox...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C30H31F3N2O5/c1-29(2,3)22-10-8-20(9-11-22)25(28(39)35-23-12-14-24(15-13-23)40-30(31,32)33)18-19-4-6-21(7-5-19)27(38)34-17-16-26(36)37/h4-15,25H,16-18H2,1-3H3,(H,34,38)(H,35,39)(H,36,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144009
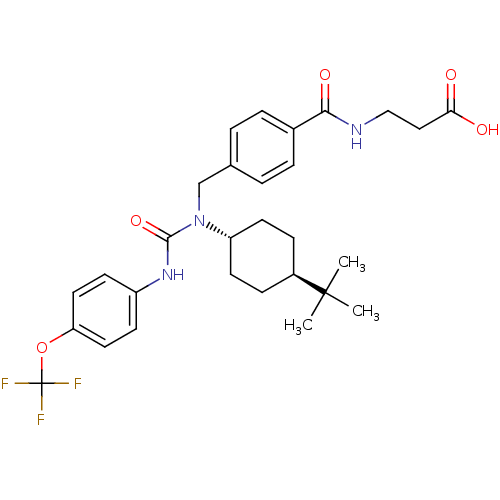
(3-{4-[1-(4-tert-Butyl-cyclohexyl)-3-(4-trifluorome...)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)N(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(OC(F)(F)F)cc1 |wU:4.3,wD:7.10,(10.18,2.9,;9.39,1.56,;8.05,2.34,;8.62,.21,;10.74,.78,;12.08,1.53,;13.41,.77,;13.41,-.77,;12.07,-1.54,;10.74,-.77,;14.74,-1.54,;14.74,-3.07,;13.39,-3.86,;12.07,-3.1,;10.74,-3.86,;10.74,-5.4,;12.07,-6.17,;13.41,-5.4,;9.41,-6.19,;9.43,-7.72,;8.06,-5.42,;6.74,-6.2,;5.4,-5.44,;4.07,-6.23,;2.73,-5.47,;4.07,-7.77,;16.06,-.77,;16.06,.77,;17.39,-1.54,;18.71,-.77,;20.04,-1.53,;21.38,-.77,;21.36,.78,;22.71,1.56,;22.7,3.09,;21.17,3.11,;24.23,3.11,;22.71,4.62,;20.04,1.55,;18.71,.77,)| Show InChI InChI=1S/C29H36F3N3O5/c1-28(2,3)21-8-12-23(13-9-21)35(27(39)34-22-10-14-24(15-11-22)40-29(30,31)32)18-19-4-6-20(7-5-19)26(38)33-17-16-25(36)37/h4-7,10-11,14-15,21,23H,8-9,12-13,16-18H2,1-3H3,(H,33,38)(H,34,39)(H,36,37)/t21-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144012
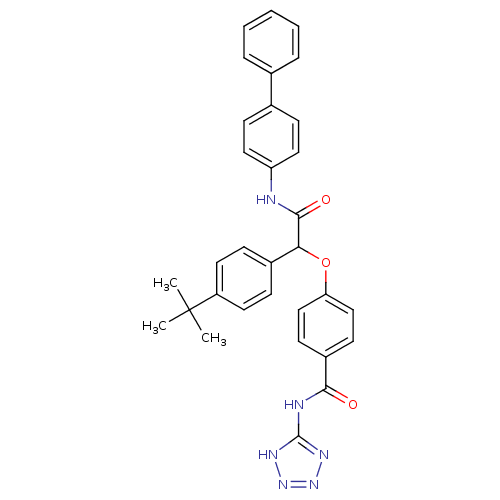
(4-[(Biphenyl-4-ylcarbamoyl)-(4-tert-butyl-phenyl)-...)Show SMILES CC(C)(C)c1ccc(cc1)C(Oc1ccc(cc1)C(=O)Nc1nnn[nH]1)C(=O)Nc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C32H30N6O3/c1-32(2,3)25-15-9-23(10-16-25)28(30(40)33-26-17-11-22(12-18-26)21-7-5-4-6-8-21)41-27-19-13-24(14-20-27)29(39)34-31-35-37-38-36-31/h4-20,28H,1-3H3,(H,33,40)(H2,34,35,36,37,38,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 1/2/3/4/5/6/7/8/9
(Homo sapiens (Human)) | BDBM50144008

(3-{4-[2-(4-Benzofuran-2-yl-phenylcarbamoyl)-2-(4-t...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C37H36N2O5/c1-37(2,3)29-16-12-25(13-17-29)31(22-24-8-10-27(11-9-24)35(42)38-21-20-34(40)41)36(43)39-30-18-14-26(15-19-30)33-23-28-6-4-5-7-32(28)44-33/h4-19,23,31H,20-22H2,1-3H3,(H,38,42)(H,39,43)(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against glucagon induced monkey adenylate cyclase |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144018
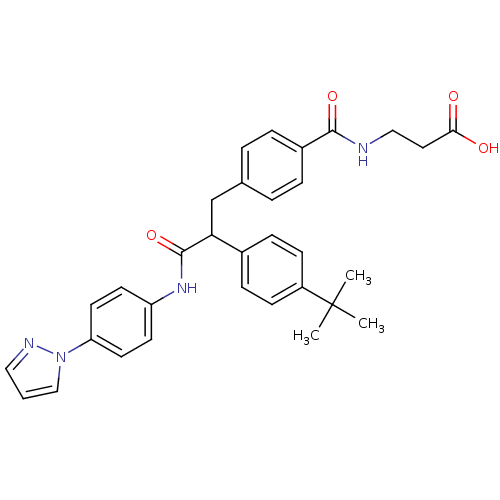
(3-{4-[2-(4-tert-Butyl-phenyl)-2-(4-pyrazol-1-yl-ph...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)-n1cccn1 Show InChI InChI=1S/C32H34N4O4/c1-32(2,3)25-11-9-23(10-12-25)28(21-22-5-7-24(8-6-22)30(39)33-19-17-29(37)38)31(40)35-26-13-15-27(16-14-26)36-20-4-18-34-36/h4-16,18,20,28H,17,19,21H2,1-3H3,(H,33,39)(H,35,40)(H,37,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144000
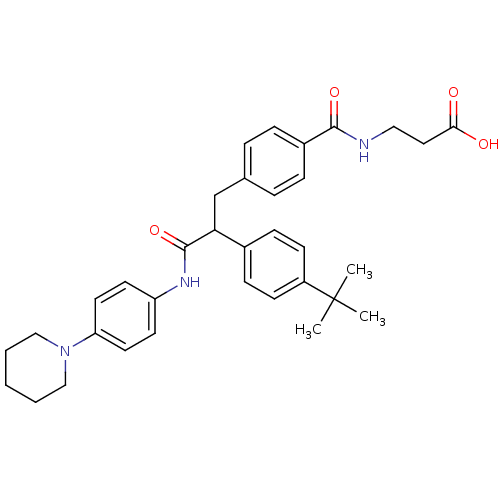
(3-{4-[2-(4-tert-Butyl-phenyl)-2-(4-piperidin-1-yl-...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C34H41N3O4/c1-34(2,3)27-13-11-25(12-14-27)30(23-24-7-9-26(10-8-24)32(40)35-20-19-31(38)39)33(41)36-28-15-17-29(18-16-28)37-21-5-4-6-22-37/h7-18,30H,4-6,19-23H2,1-3H3,(H,35,40)(H,36,41)(H,38,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144007
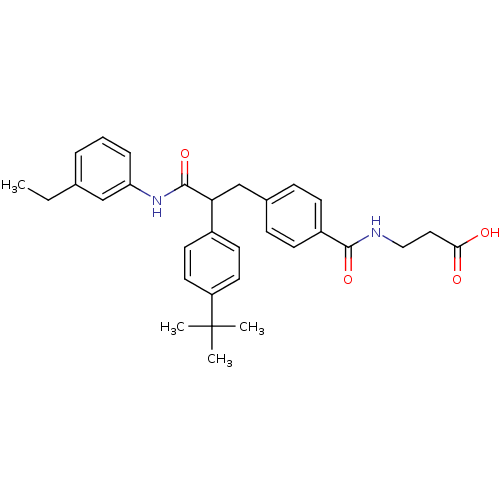
(3-{4-[2-(4-tert-Butyl-phenyl)-2-(3-ethyl-phenylcar...)Show SMILES CCc1cccc(NC(=O)C(Cc2ccc(cc2)C(=O)NCCC(O)=O)c2ccc(cc2)C(C)(C)C)c1 Show InChI InChI=1S/C31H36N2O4/c1-5-21-7-6-8-26(19-21)33-30(37)27(23-13-15-25(16-14-23)31(2,3)4)20-22-9-11-24(12-10-22)29(36)32-18-17-28(34)35/h6-16,19,27H,5,17-18,20H2,1-4H3,(H,32,36)(H,33,37)(H,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human glucagon receptor (h-GlucR) was determined |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183965
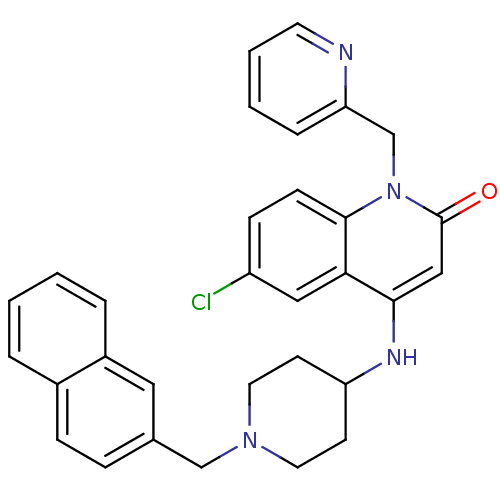
(6-chloro-4-(1-(naphthalen-2-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2c1 Show InChI InChI=1S/C31H29ClN4O/c32-25-10-11-30-28(18-25)29(19-31(37)36(30)21-27-7-3-4-14-33-27)34-26-12-15-35(16-13-26)20-22-8-9-23-5-1-2-6-24(23)17-22/h1-11,14,17-19,26,34H,12-13,15-16,20-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 1/2/3/4/5/6/7/8/9
(Homo sapiens (Human)) | BDBM50144003

(3-{4-[2-(4-tert-Butyl-phenyl)-2-(4-trifluoromethox...)Show SMILES CC(C)(C)c1ccc(cc1)C(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C30H31F3N2O5/c1-29(2,3)22-10-8-20(9-11-22)25(28(39)35-23-12-14-24(15-13-23)40-30(31,32)33)18-19-4-6-21(7-5-19)27(38)34-17-16-26(36)37/h4-15,25H,16-18H2,1-3H3,(H,34,38)(H,35,39)(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against glucagon induced human adenylate cyclase |
Bioorg Med Chem Lett 14: 2047-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.056
BindingDB Entry DOI: 10.7270/Q2XS5TTH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data