 Found 94 hits with Last Name = 'nixon' and Initial = 'c'
Found 94 hits with Last Name = 'nixon' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607602

(CHEMBL5220994)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CC(F)(F)C1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607596

(CHEMBL5221053)Show SMILES Clc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607597
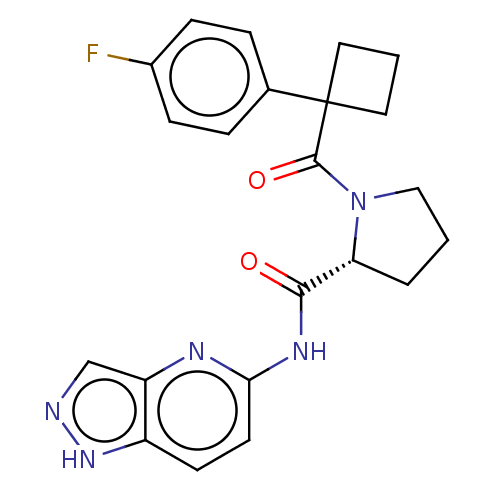
(CHEMBL5219157)Show SMILES Fc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607595

(CHEMBL5219030)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ccc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607593
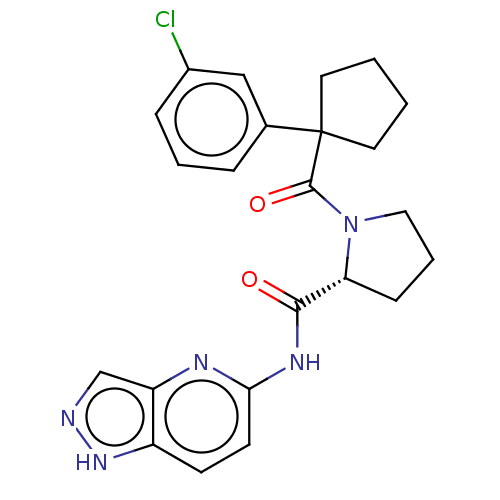
(CHEMBL5219693)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607601
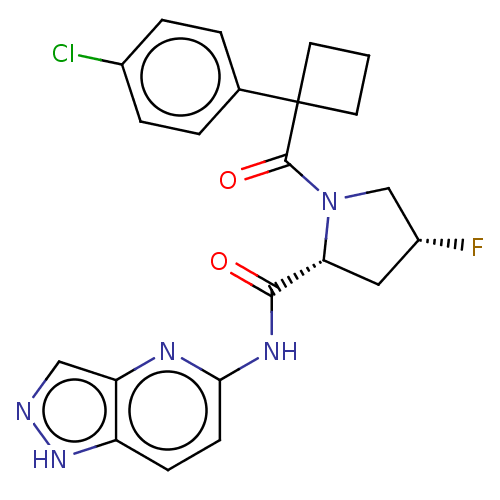
(CHEMBL5219667)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CCC1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607599
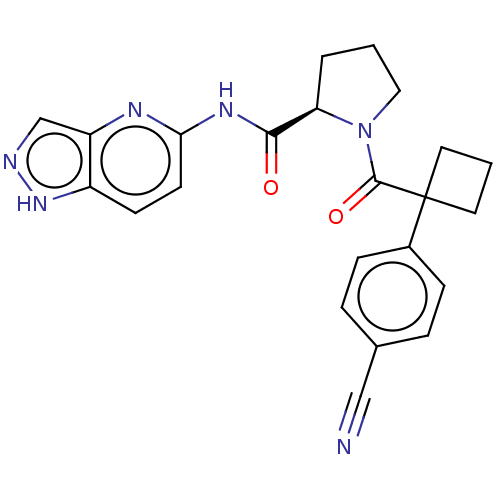
(CHEMBL5220732)Show SMILES O=C(Nc1ccc2[nH]ncc2n1)[C@H]1CCCN1C(=O)C1(CCC1)c1ccc(cc1)C#N |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607598
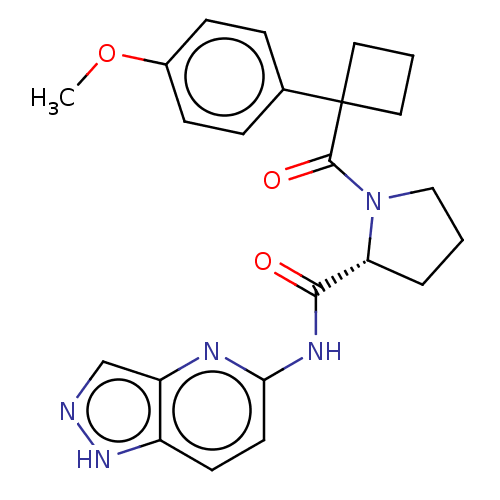
(CHEMBL5220447)Show SMILES COc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607589
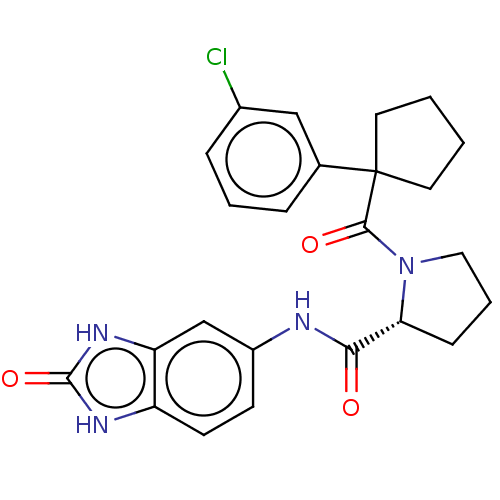
(CHEMBL5219678)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]c(=O)[nH]c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607585
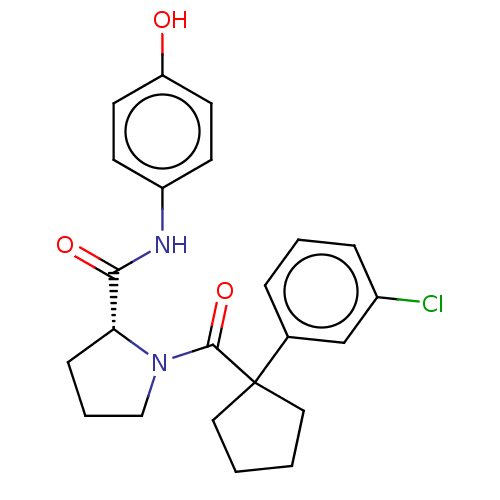
(CHEMBL5219512)Show SMILES Oc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607588
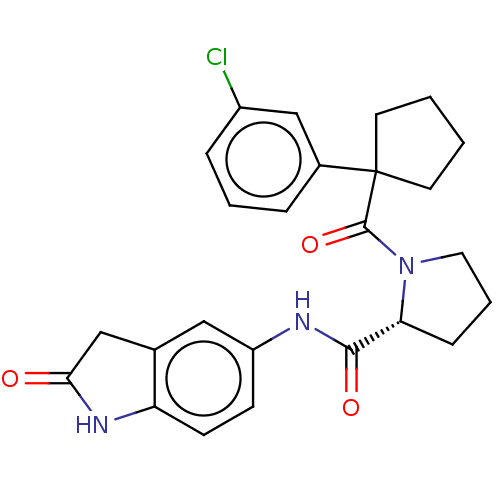
(CHEMBL5220546)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2NC(=O)Cc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607586
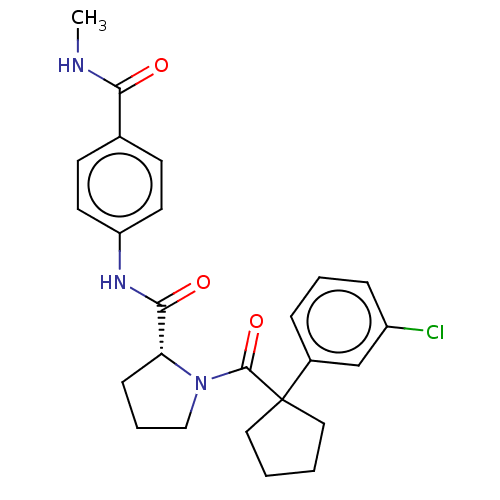
(CHEMBL5221030)Show SMILES CNC(=O)c1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607590
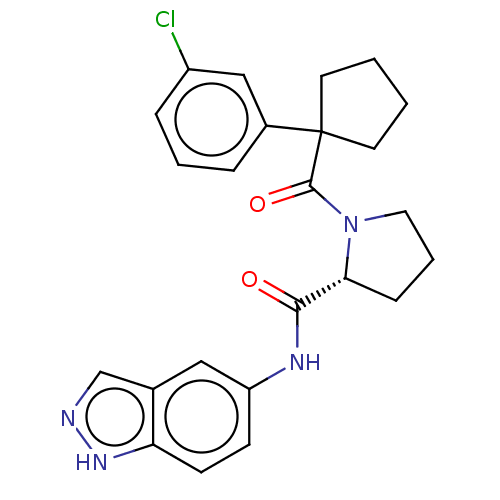
(CHEMBL5220332)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607605
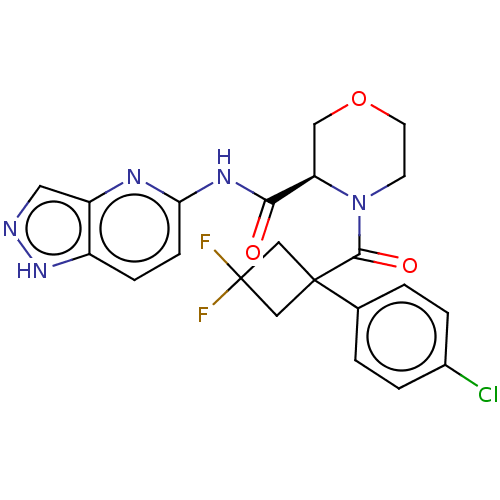
(CHEMBL5219177)Show SMILES FC1(F)CC(C1)(C(=O)N1CCOC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607592
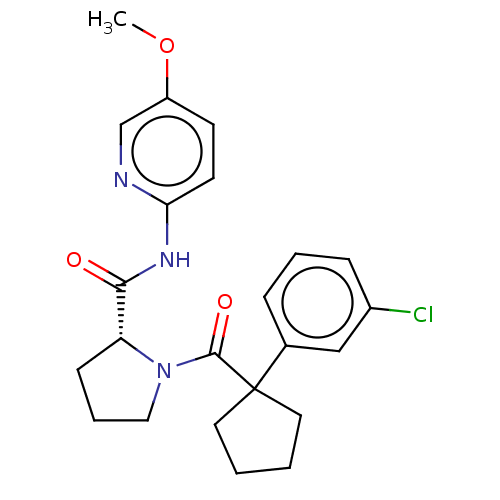
(CHEMBL5219466)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)nc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607584
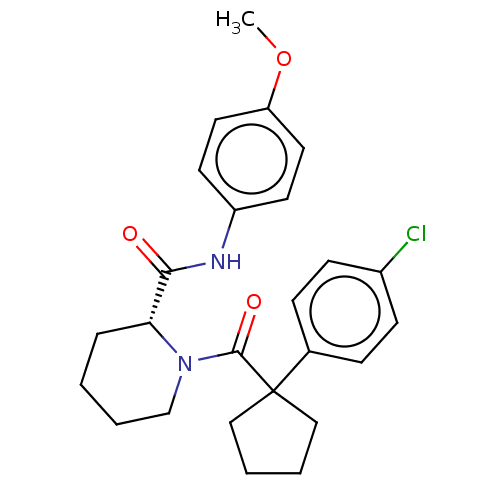
(CHEMBL5221088)Show SMILES COc1ccc(NC(=O)[C@H]2CCCCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607577
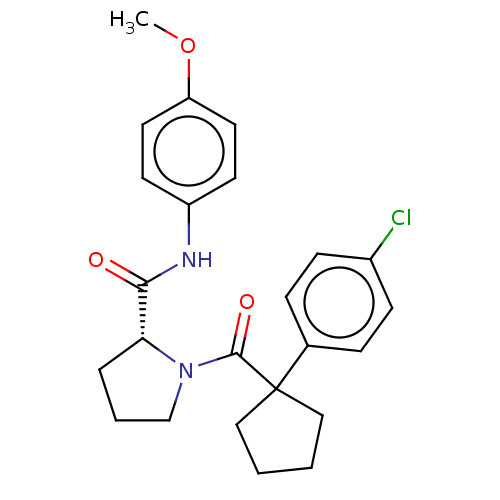
(CHEMBL5219851)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607580
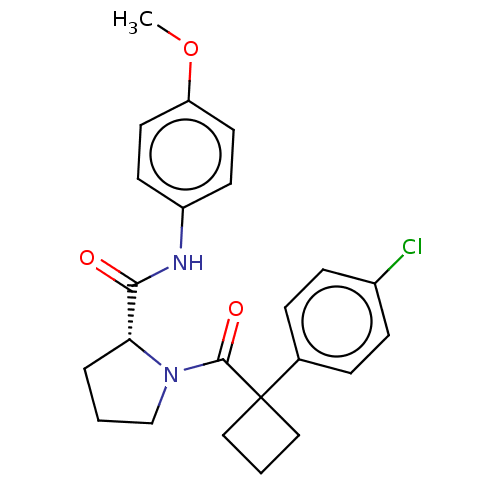
(CHEMBL5218875)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607604
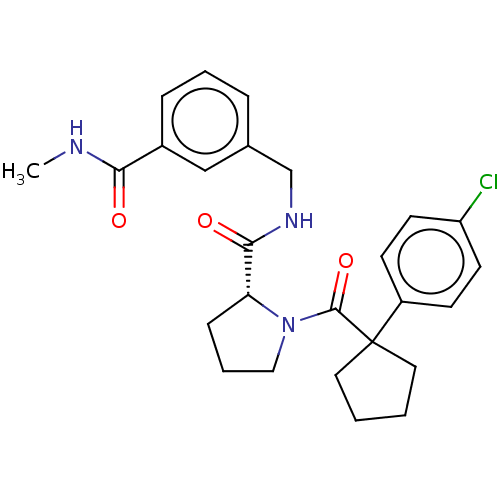
(CHEMBL5219712)Show SMILES CNC(=O)c1cccc(CNC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607594
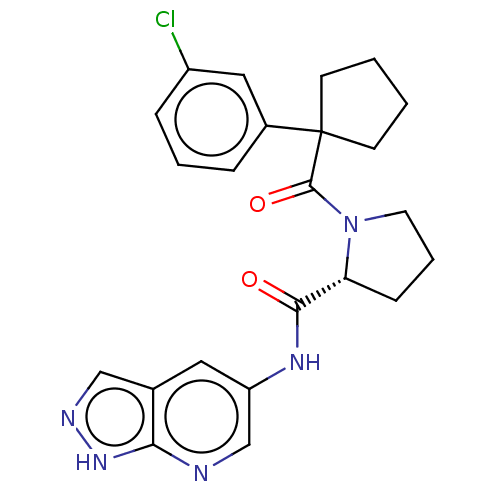
(CHEMBL5219204)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1cnc2[nH]ncc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607591
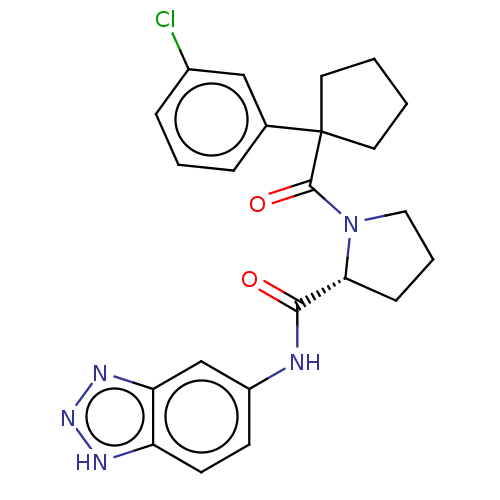
(CHEMBL5219472)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]nnc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607581
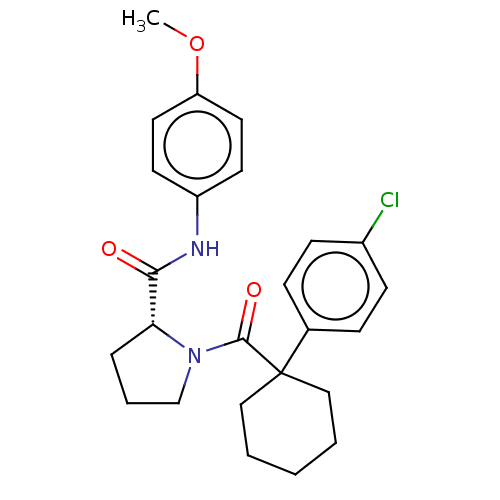
(CHEMBL5221117)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607583
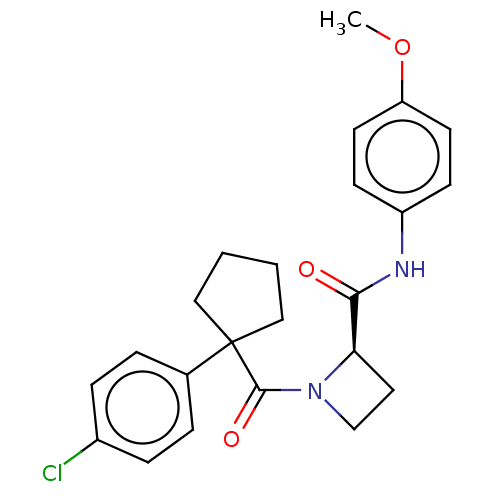
(CHEMBL5218882)Show SMILES COc1ccc(NC(=O)[C@H]2CCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510295
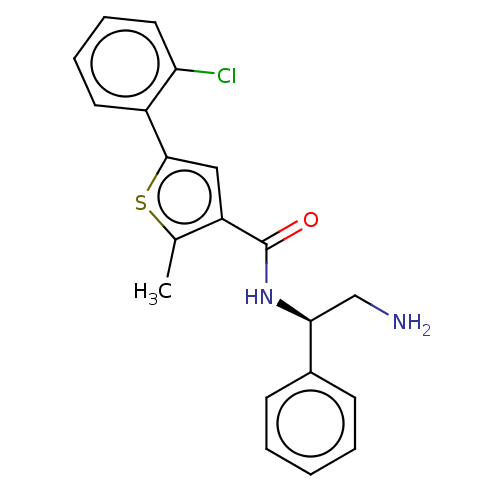
(CHEMBL4443422)Show SMILES Cc1sc(cc1C(=O)N[C@@H](CN)c1ccccc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C20H19ClN2OS/c1-13-16(11-19(25-13)15-9-5-6-10-17(15)21)20(24)23-18(12-22)14-7-3-2-4-8-14/h2-11,18H,12,22H2,1H3,(H,23,24)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607582
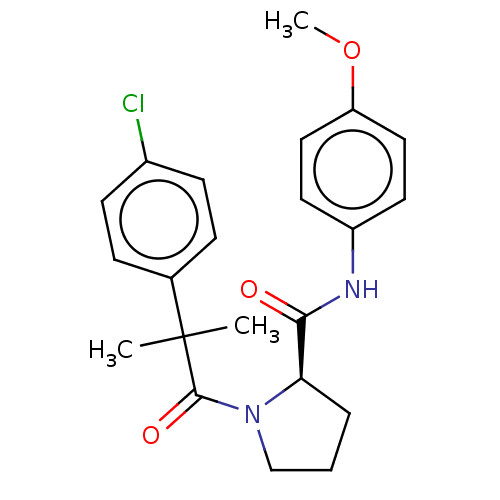
(CHEMBL5220135)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C(C)(C)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607578
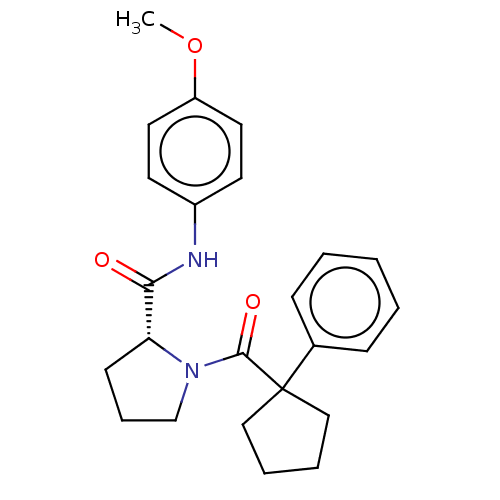
(CHEMBL5218485)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2ccccc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607579
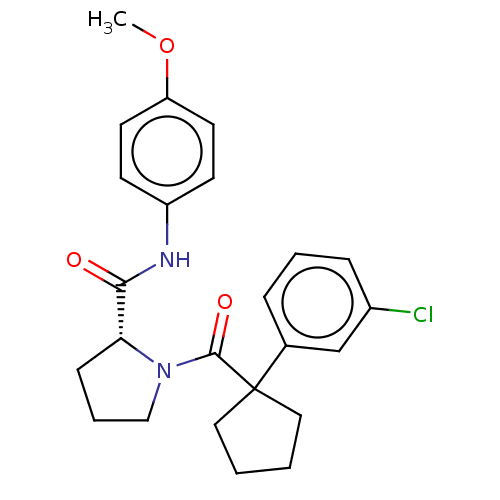
(CHEMBL5219575)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607603
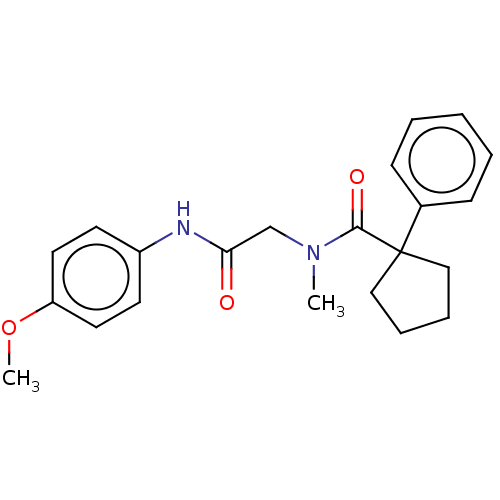
(CHEMBL1700017)Show SMILES COc1ccc(NC(=O)CN(C)C(=O)C2(CCCC2)c2ccccc2)cc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510294
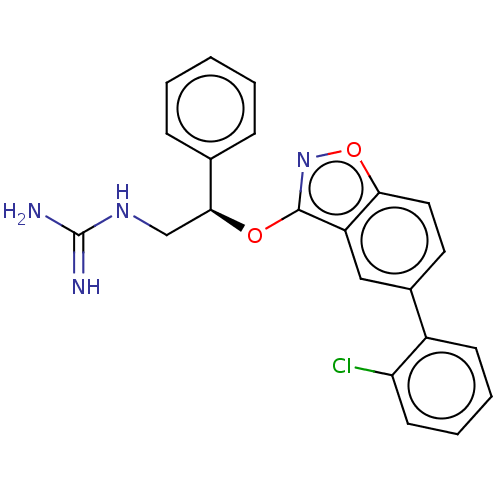
(CHEMBL4592805)Show SMILES NC(=N)NC[C@H](Oc1noc2ccc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C22H19ClN4O2/c23-18-9-5-4-8-16(18)15-10-11-19-17(12-15)21(27-29-19)28-20(13-26-22(24)25)14-6-2-1-3-7-14/h1-12,20H,13H2,(H4,24,25,26)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510288
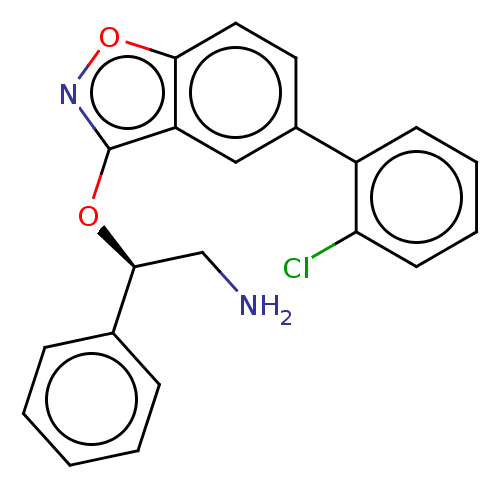
(CHEMBL4445556)Show SMILES NC[C@H](Oc1noc2ccc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C21H17ClN2O2/c22-18-9-5-4-8-16(18)15-10-11-19-17(12-15)21(24-26-19)25-20(13-23)14-6-2-1-3-7-14/h1-12,20H,13,23H2/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510286
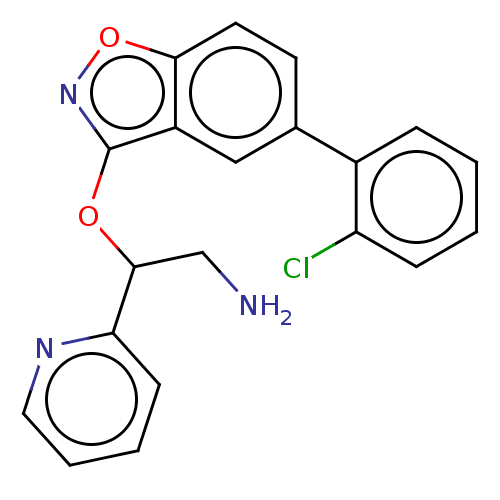
(CHEMBL4467227)Show InChI InChI=1S/C20H16ClN3O2/c21-16-6-2-1-5-14(16)13-8-9-18-15(11-13)20(24-26-18)25-19(12-22)17-7-3-4-10-23-17/h1-11,19H,12,22H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510292
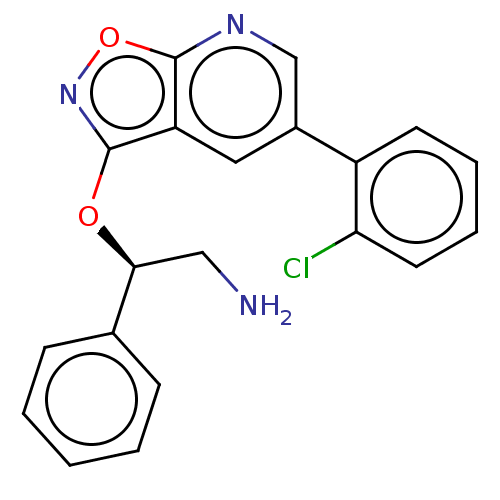
(CHEMBL4471339)Show SMILES NC[C@H](Oc1noc2ncc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C20H16ClN3O2/c21-17-9-5-4-8-15(17)14-10-16-19(23-12-14)26-24-20(16)25-18(11-22)13-6-2-1-3-7-13/h1-10,12,18H,11,22H2/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607587
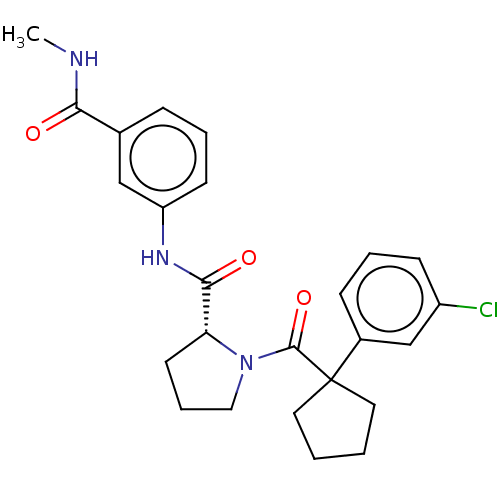
(CHEMBL5219253)Show SMILES CNC(=O)c1cccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510293
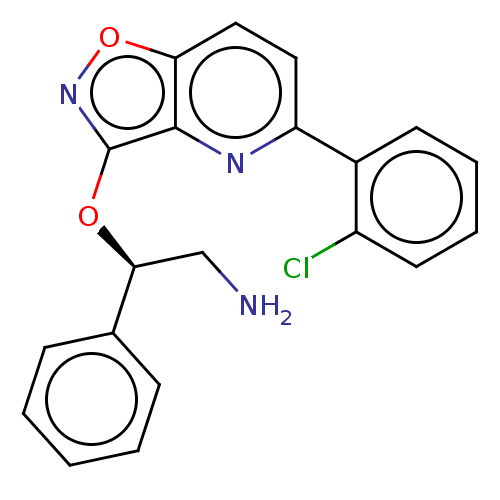
(CHEMBL4528547)Show SMILES NC[C@H](Oc1noc2ccc(nc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C20H16ClN3O2/c21-15-9-5-4-8-14(15)16-10-11-17-19(23-16)20(24-26-17)25-18(12-22)13-6-2-1-3-7-13/h1-11,18H,12,22H2/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510289
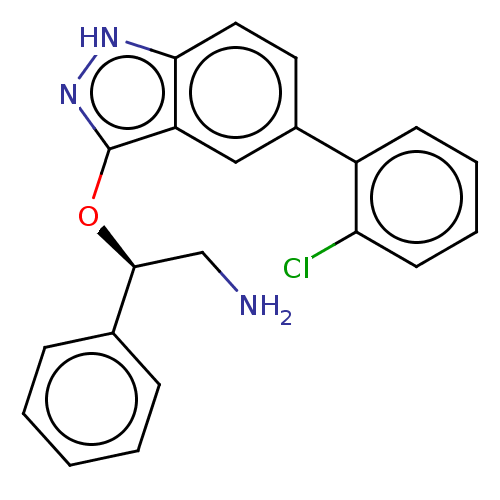
(CHEMBL4588294)Show SMILES NC[C@H](Oc1n[nH]c2ccc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C21H18ClN3O/c22-18-9-5-4-8-16(18)15-10-11-19-17(12-15)21(25-24-19)26-20(13-23)14-6-2-1-3-7-14/h1-12,20H,13,23H2,(H,24,25)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510287
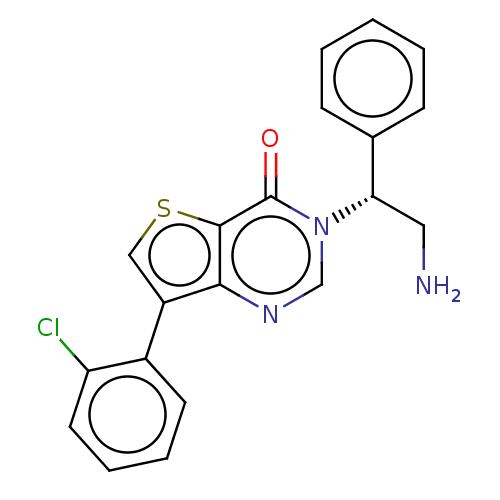
(CHEMBL4468900)Show SMILES NC[C@@H](c1ccccc1)n1cnc2c(csc2c1=O)-c1ccccc1Cl |r| Show InChI InChI=1S/C20H16ClN3OS/c21-16-9-5-4-8-14(16)15-11-26-19-18(15)23-12-24(20(19)25)17(10-22)13-6-2-1-3-7-13/h1-9,11-12,17H,10,22H2/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510290
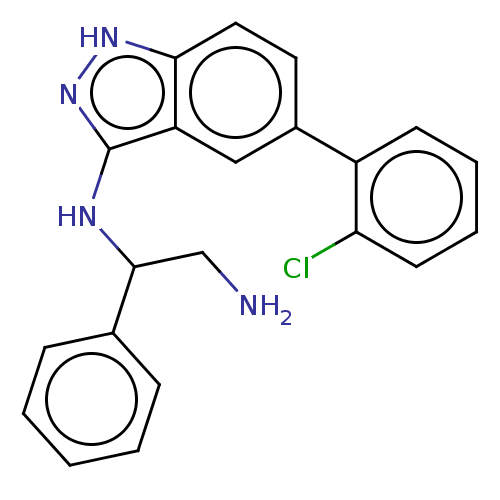
(CHEMBL4583055)Show SMILES NCC(Nc1n[nH]c2ccc(cc12)-c1ccccc1Cl)c1ccccc1 Show InChI InChI=1S/C21H19ClN4/c22-18-9-5-4-8-16(18)15-10-11-19-17(12-15)21(26-25-19)24-20(13-23)14-6-2-1-3-7-14/h1-12,20H,13,23H2,(H2,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50510293

(CHEMBL4528547)Show SMILES NC[C@H](Oc1noc2ccc(nc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C20H16ClN3O2/c21-15-9-5-4-8-14(15)16-10-11-17-19(23-16)20(24-26-17)25-18(12-22)13-6-2-1-3-7-13/h1-11,18H,12,22H2/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by QPatch assay |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50613723
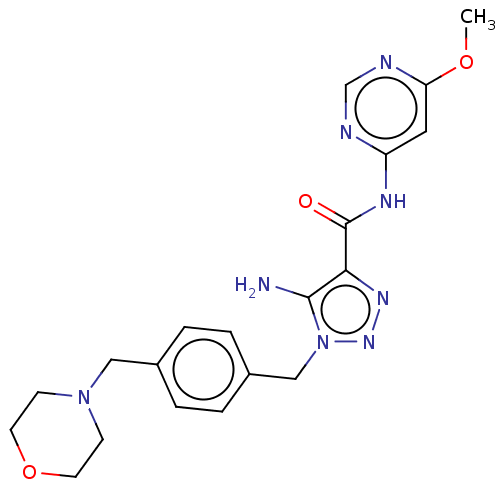
(CHEMBL5268330)Show SMILES COc1cc(NC(=O)c2nnn(Cc3ccc(CN4CCOCC4)cc3)c2N)ncn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510296
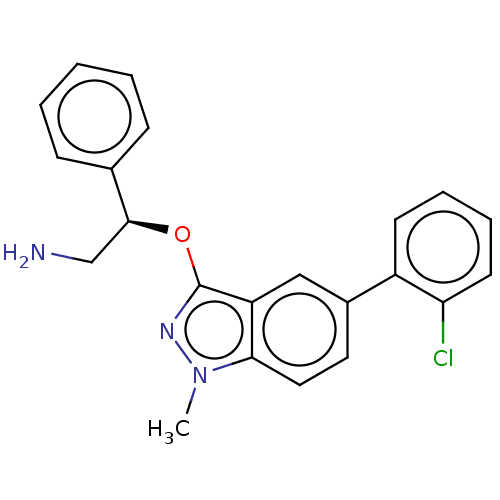
(CHEMBL4592804)Show SMILES Cn1nc(O[C@@H](CN)c2ccccc2)c2cc(ccc12)-c1ccccc1Cl |r| Show InChI InChI=1S/C22H20ClN3O/c1-26-20-12-11-16(17-9-5-6-10-19(17)23)13-18(20)22(25-26)27-21(14-24)15-7-3-2-4-8-15/h2-13,21H,14,24H2,1H3/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50613719
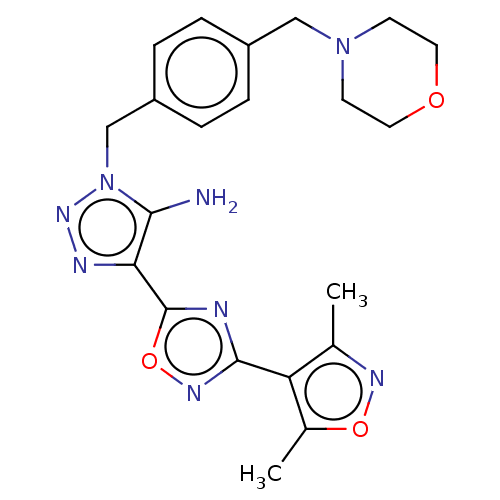
(CHEMBL5281165)Show SMILES Cc1noc(C)c1-c1noc(n1)-c1nnn(Cc2ccc(CN3CCOCC3)cc2)c1N | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50510295

(CHEMBL4443422)Show SMILES Cc1sc(cc1C(=O)N[C@@H](CN)c1ccccc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C20H19ClN2OS/c1-13-16(11-19(25-13)15-9-5-6-10-17(15)21)20(24)23-18(12-22)14-7-3-2-4-8-14/h2-11,18H,12,22H2,1H3,(H,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by QPatch assay |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50613721
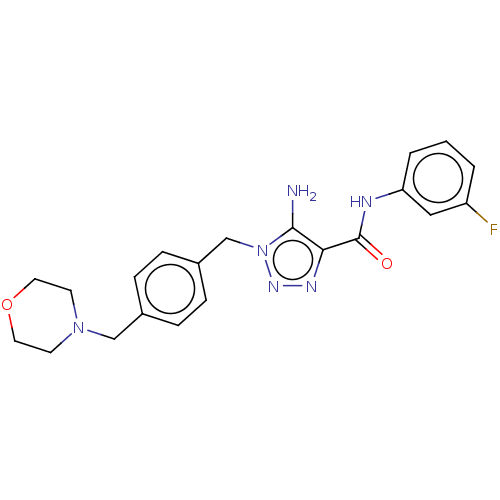
(CHEMBL5275547)Show SMILES Nc1c(nnn1Cc1ccc(CN2CCOCC2)cc1)C(=O)Nc1cccc(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510291
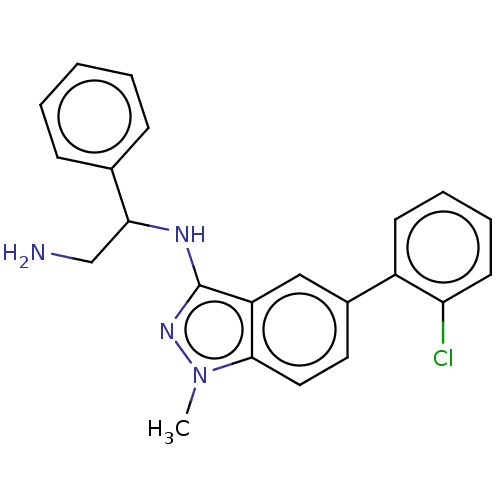
(CHEMBL4553177)Show SMILES Cn1nc(NC(CN)c2ccccc2)c2cc(ccc12)-c1ccccc1Cl Show InChI InChI=1S/C22H21ClN4/c1-27-21-12-11-16(17-9-5-6-10-19(17)23)13-18(21)22(26-27)25-20(14-24)15-7-3-2-4-8-15/h2-13,20H,14,24H2,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50510294

(CHEMBL4592805)Show SMILES NC(=N)NC[C@H](Oc1noc2ccc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C22H19ClN4O2/c23-18-9-5-4-8-16(18)15-10-11-19-17(12-15)21(27-29-19)28-20(13-26-22(24)25)14-6-2-1-3-7-14/h1-12,20H,13H2,(H4,24,25,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by QPatch assay |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510297
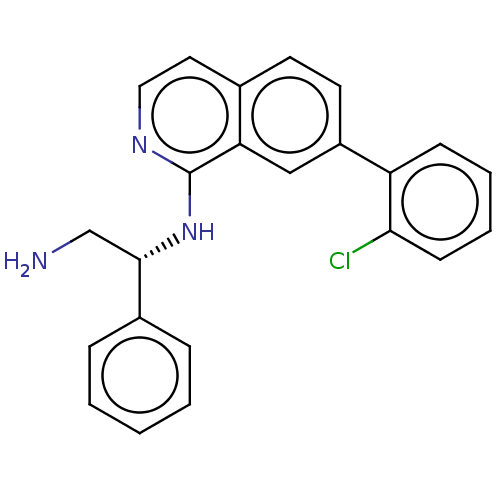
(CHEMBL4577405)Show SMILES NC[C@H](Nc1nccc2ccc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C23H20ClN3/c24-21-9-5-4-8-19(21)18-11-10-16-12-13-26-23(20(16)14-18)27-22(15-25)17-6-2-1-3-7-17/h1-14,22H,15,25H2,(H,26,27)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50613725
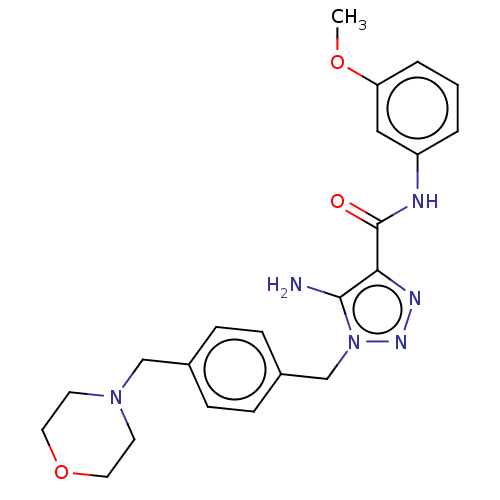
(CHEMBL5268278)Show SMILES COc1cccc(NC(=O)c2nnn(Cc3ccc(CN4CCOCC4)cc3)c2N)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50613725

(CHEMBL5268278)Show SMILES COc1cccc(NC(=O)c2nnn(Cc3ccc(CN4CCOCC4)cc3)c2N)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data