

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279 (CHEMBL4436207) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Binding affinity to wild type HIV1 protease | J Med Chem 59: 2849-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b00497 BindingDB Entry DOI: 10.7270/Q2JH3P3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737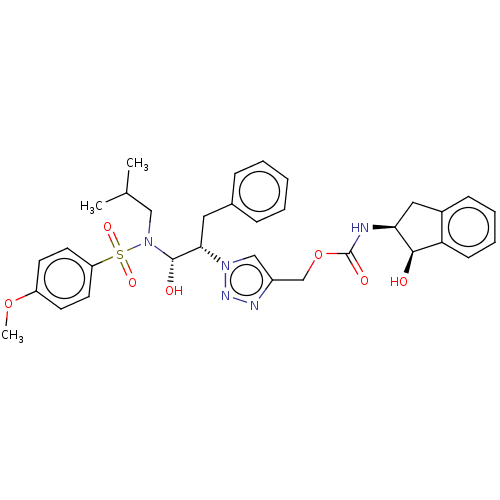 (CHEMBL3799941) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease after 48 hrs by micro-titer plate assay | J Med Chem 59: 2849-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b00497 BindingDB Entry DOI: 10.7270/Q2JH3P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737 (CHEMBL3799941) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737 (CHEMBL3799941) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Pr SF-2-WTQ7K-Pr as substrate after 24 hrs by LC/MS-MS analysis | J Med Chem 59: 2849-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b00497 BindingDB Entry DOI: 10.7270/Q2JH3P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750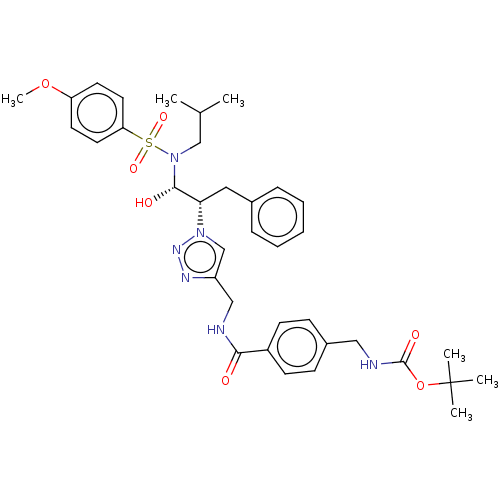 (CHEMBL3797292) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750 (CHEMBL3797292) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease after 48 hrs by micro-titer plate assay | J Med Chem 59: 2849-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b00497 BindingDB Entry DOI: 10.7270/Q2JH3P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50200943 (CHEMBL269769 | cyclopentyl (2S,3S)-3-[4-([4-(5-chl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease after 48 hrs by micro-titer plate assay | J Med Chem 59: 2849-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b00497 BindingDB Entry DOI: 10.7270/Q2JH3P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50505281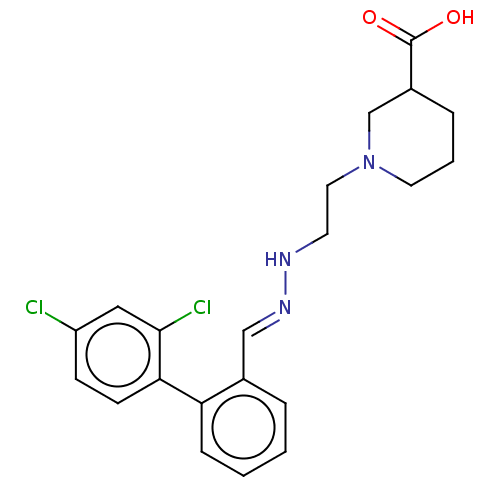 (CHEMBL2315948) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of NO711 binding to mouse GAT1 receptor expressed in HEK293 cell membranes incubated for 1 hr by LC-ESI-MS/MS analysis | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750 (CHEMBL3797292) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease G48V mutant | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737 (CHEMBL3799941) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease V82F mutant | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750 (CHEMBL3797292) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease V82F mutant | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163752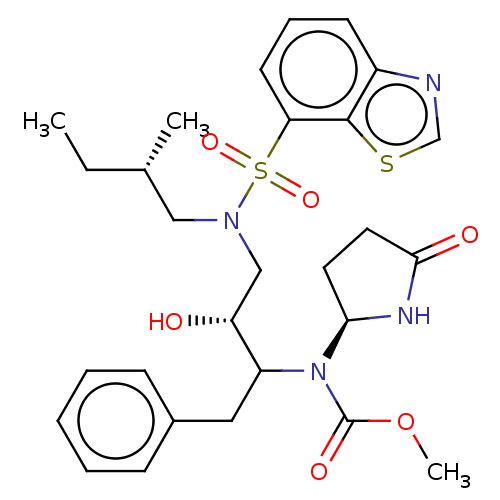 (CHEMBL3799500) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Binding affinity to wild type HIV1 protease | J Med Chem 59: 2849-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b00497 BindingDB Entry DOI: 10.7270/Q2JH3P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737 (CHEMBL3799941) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease G48V mutant | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737 (CHEMBL3799941) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease V82A mutant | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750 (CHEMBL3797292) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease V82A mutant | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Rattus norvegicus) | BDBM50505274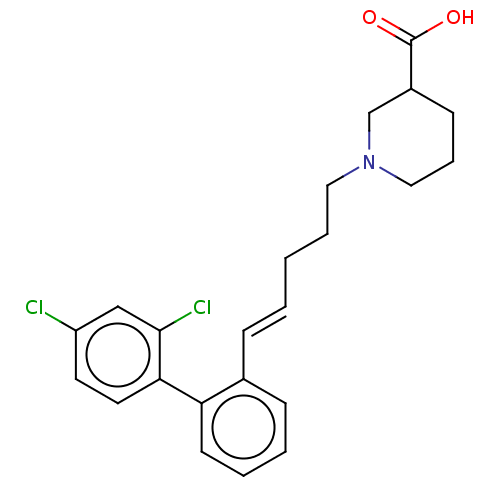 (CHEMBL2315639) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of NO711 binding to mouse GAT1 receptor expressed in HEK293 cell membranes incubated for 1 hr by LC-ESI-MS/MS analysis | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484340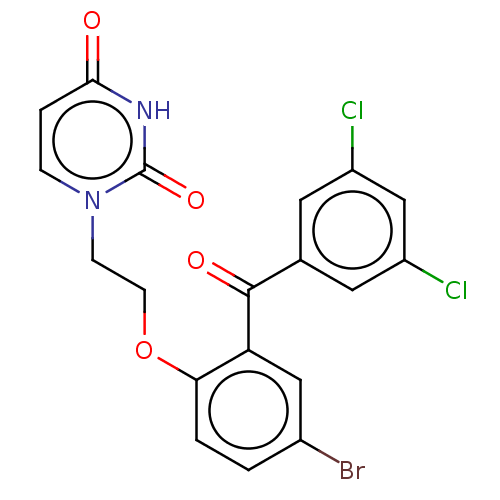 (CHEMBL1835512) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484329 (CHEMBL1835505) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490649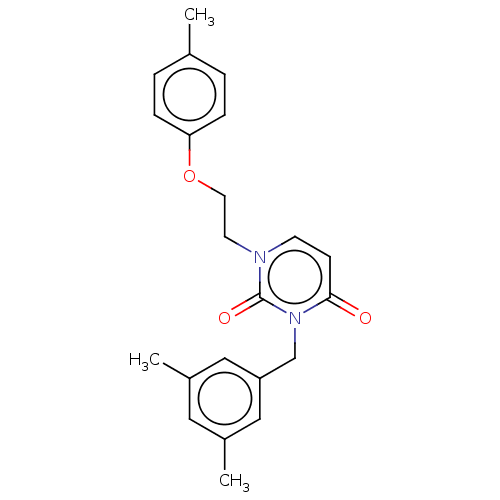 (CHEMBL2337184) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490643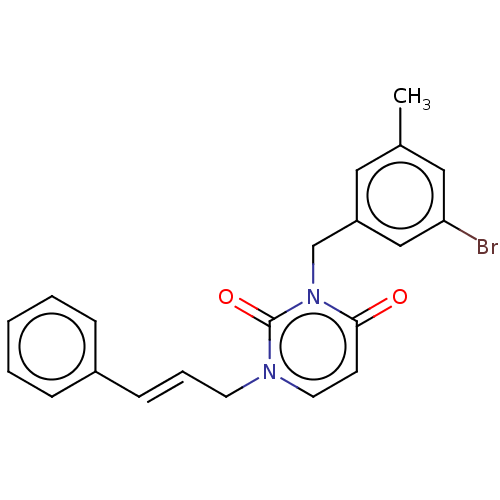 (CHEMBL2337196) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490644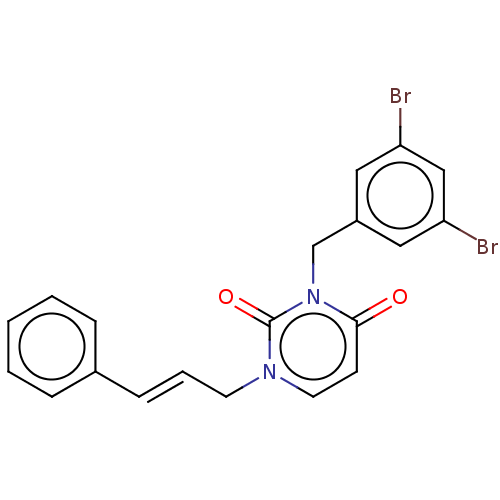 (CHEMBL2337195) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484339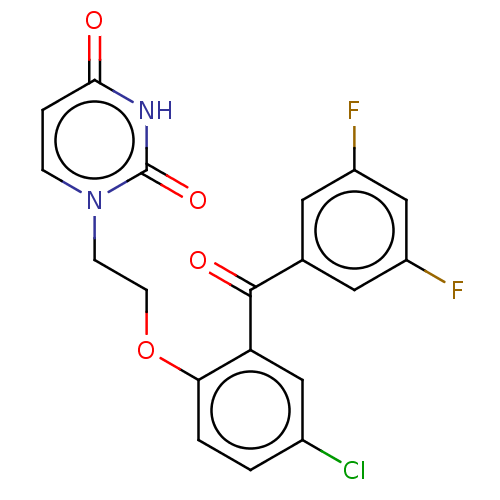 (CHEMBL1835509) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484334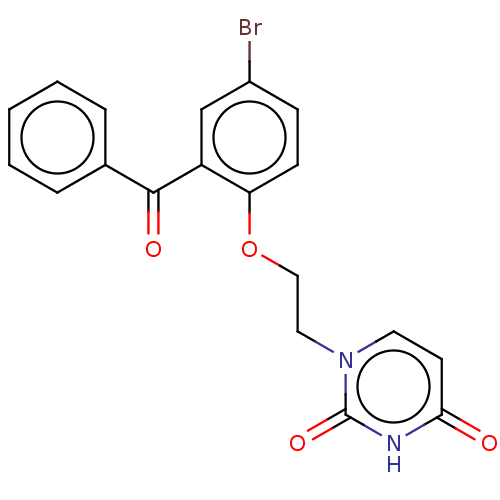 (CHEMBL1835504) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484342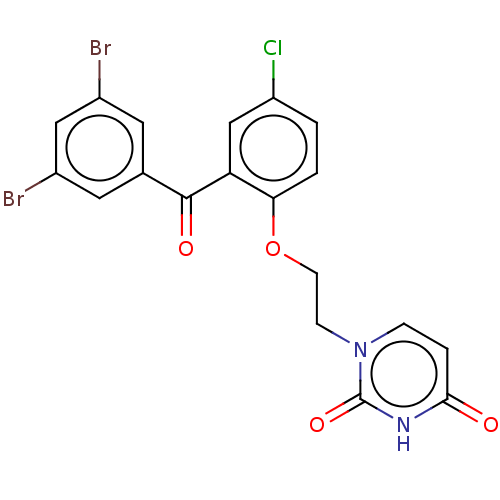 (CHEMBL1835511) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484330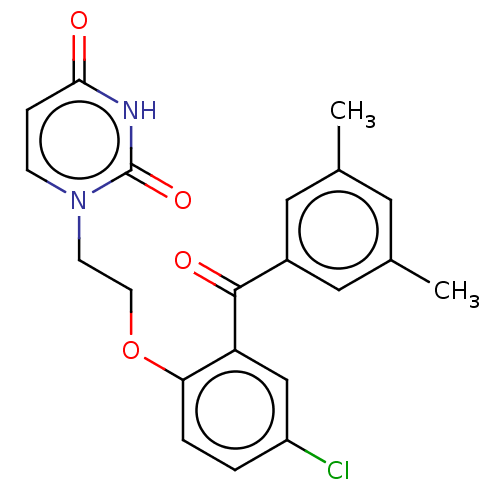 (CHEMBL1835507) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490669 (CHEMBL2337192) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484328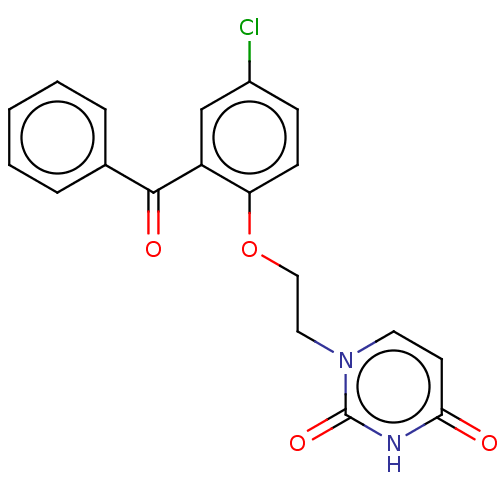 (CHEMBL1835503) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490662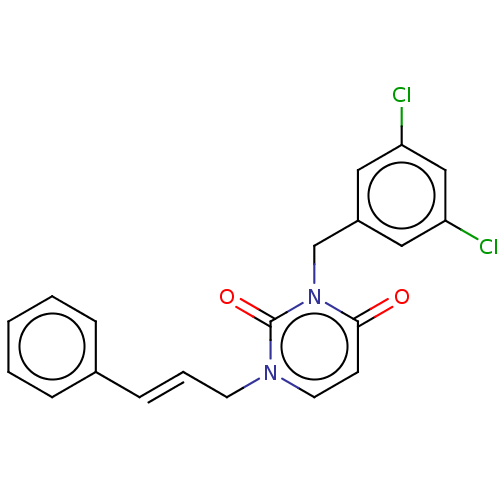 (CHEMBL2337194) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484343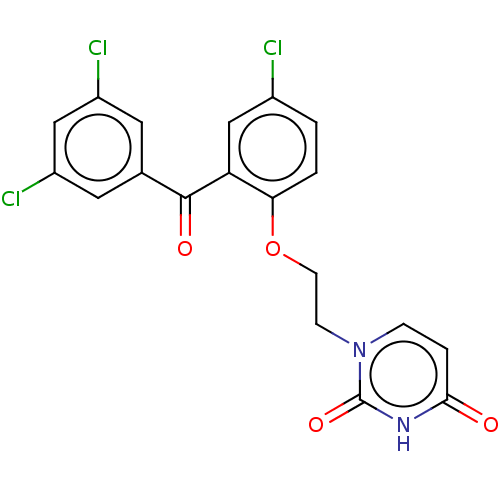 (CHEMBL1835510) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490648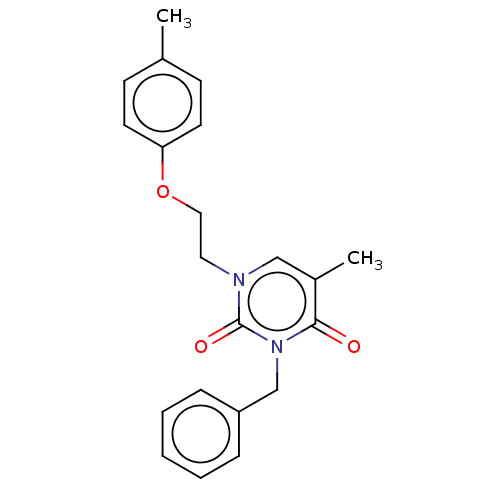 (CHEMBL2337186) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490646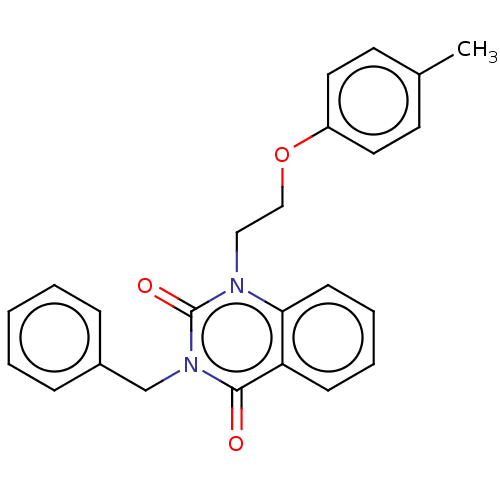 (CHEMBL2337190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490668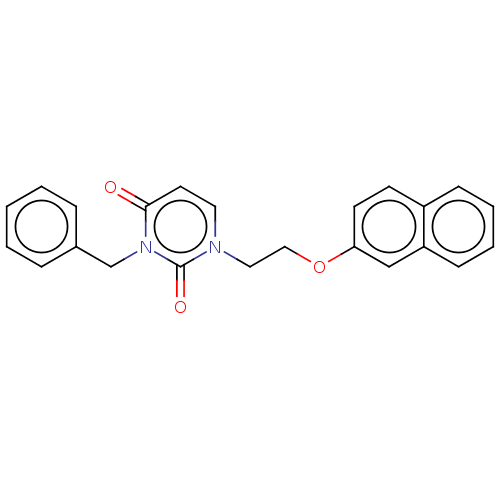 (CHEMBL2337189) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484332 (CHEMBL1835514) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484331 (CHEMBL1835508) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484337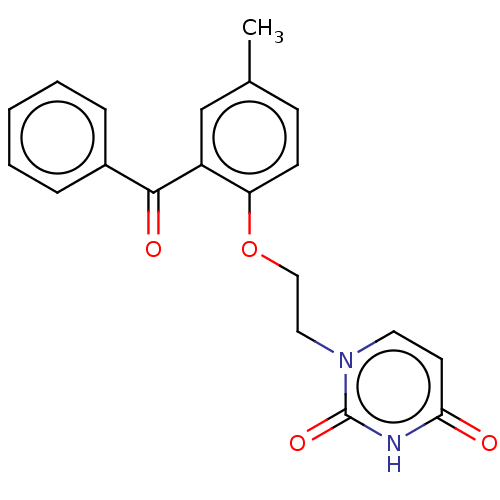 (CHEMBL1835501) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484324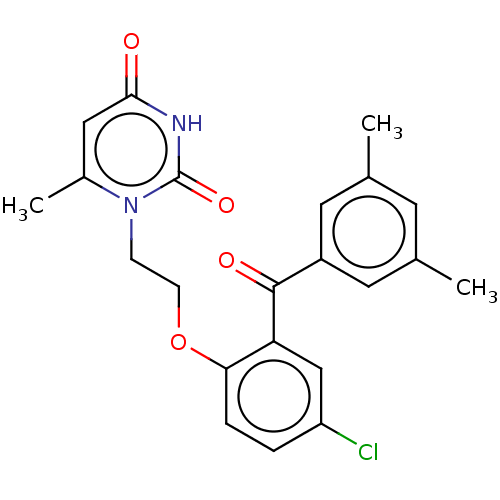 (CHEMBL1835515) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484338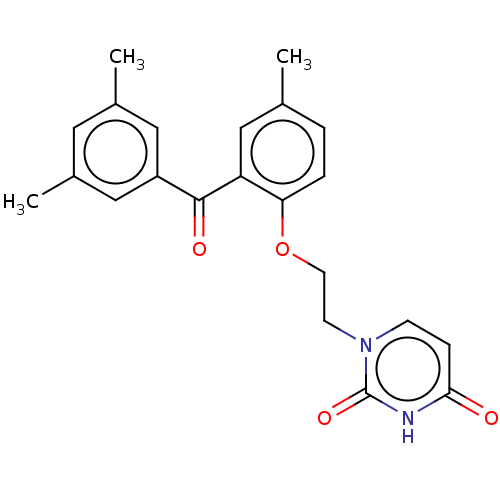 (CHEMBL1835506) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484325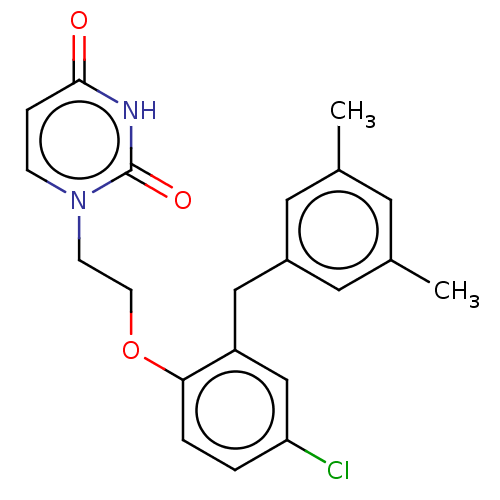 (CHEMBL1835499) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484327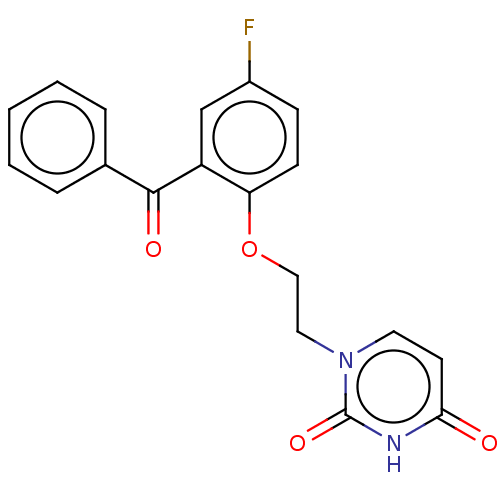 (CHEMBL1835502) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484336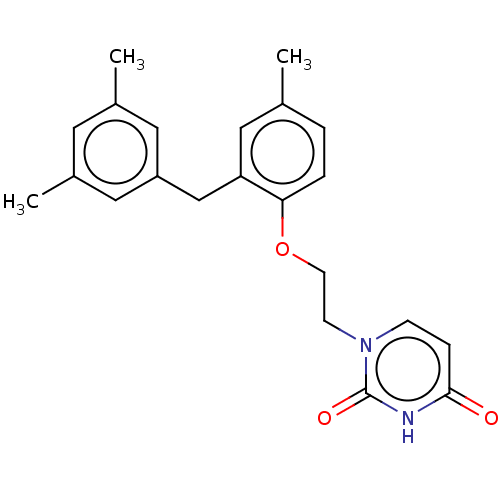 (CHEMBL1835498) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484326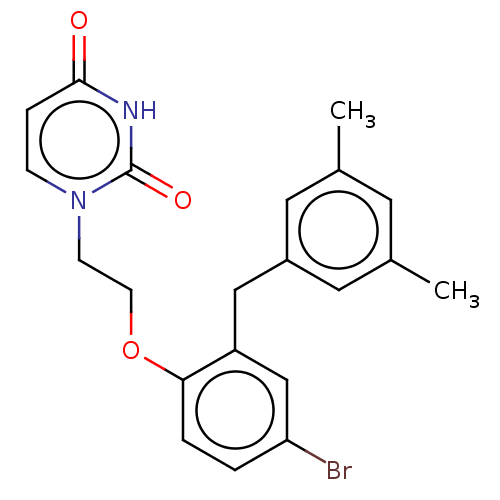 (CHEMBL1835500) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50063002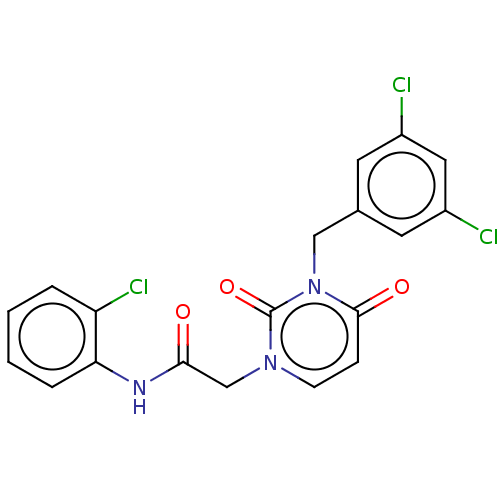 (CHEMBL3398101) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase p66/p51 pre-incubated with compound before enzyme addition using poly(rA) template and... | Bioorg Med Chem 23: 1069-81 (2015) Article DOI: 10.1016/j.bmc.2015.01.002 BindingDB Entry DOI: 10.7270/Q2RR20XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484341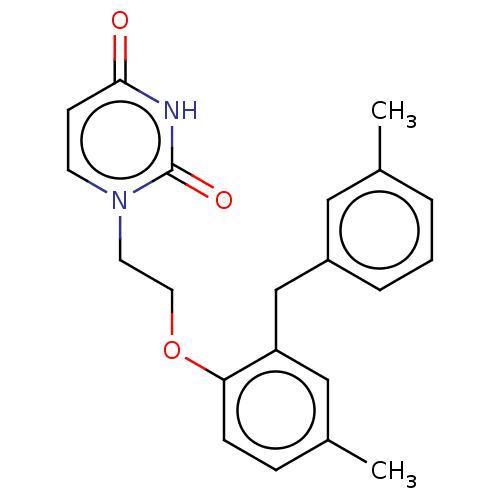 (CHEMBL1835497) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490660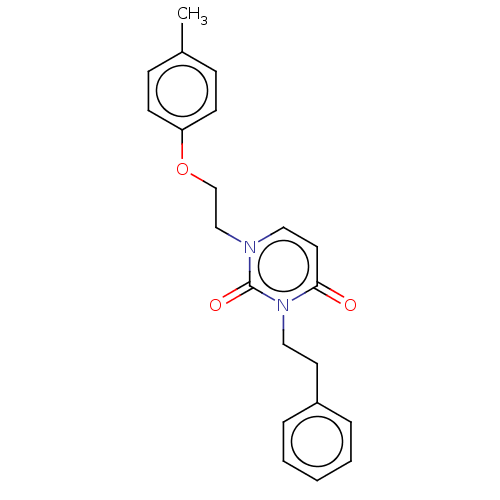 (CHEMBL2337187) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490642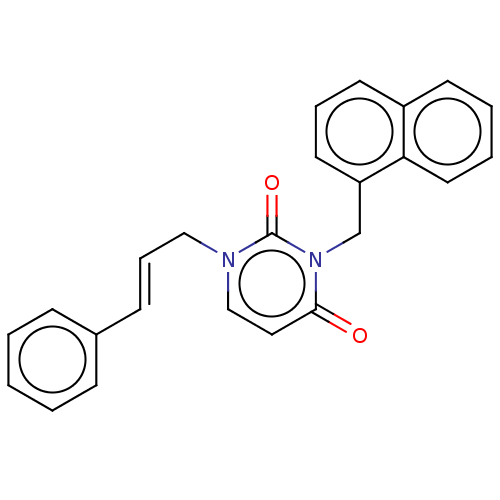 (CHEMBL2337197) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490665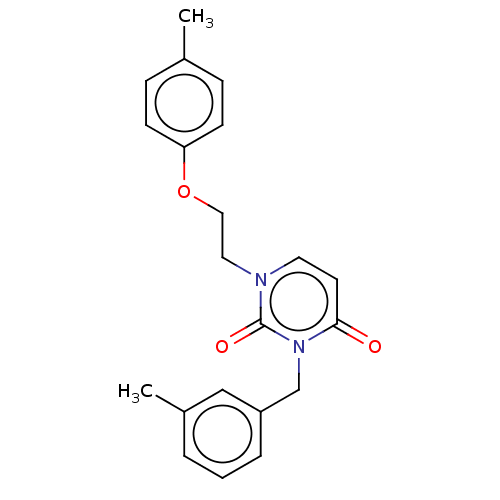 (CHEMBL2337181) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 1150-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.027 BindingDB Entry DOI: 10.7270/Q2NZ8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2615 total ) | Next | Last >> |