

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM403740 (6-Chloro-7-((3-(cyclopropylmethyl)-7-methyl-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400239 (7-((1-(2-Cyclopropylethyl)-5-fluoro-1H-benzo[d]imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400243 (6-((3-(Cyclopropylmethyl)-7-methyl-3H-imidazo[4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400238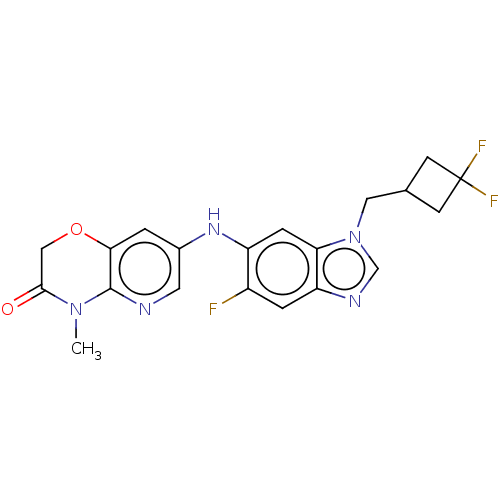 (7-((1-((3,3-Difluorocyclobutyl)methyl)-5-fluoro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400240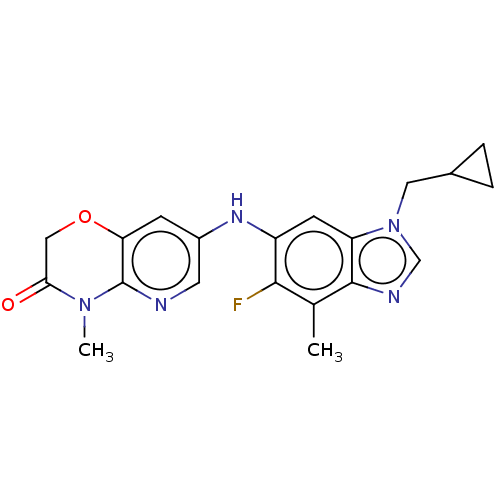 (7-((1-(Cyclopropylmethyl)-5-fluoro-4-methyl-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400242 (N-(6-(1H-Imidazol-1-yl)pyridin-3-yl)-3-(cyclopropy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400234 (7-((1-(Cyclopropylmethyl)-1H-imidazo[4,5-b]pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400241 (7-((3-(Cyclopropylmethyl)-3H-imidazo[4,5-b]pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400236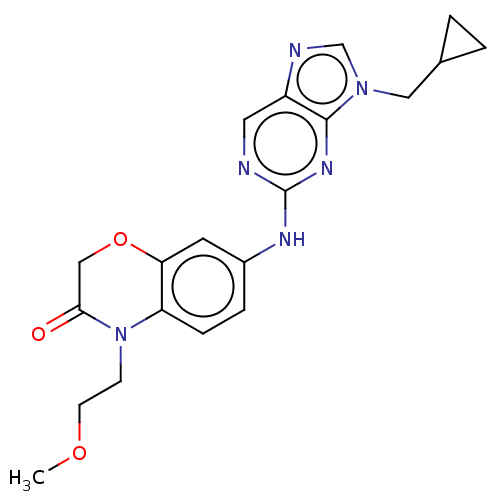 (7-((9-(Cyclopropylmethyl)-9H-purin-2-yl)amino)-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400233 (4-Cyclopropyl-7-((1-(cyclopropylmethyl)-1H-imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 38.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400235 (6-((1-(Cyclopropylmethyl)-1H-imidazo[4,5-b]pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297677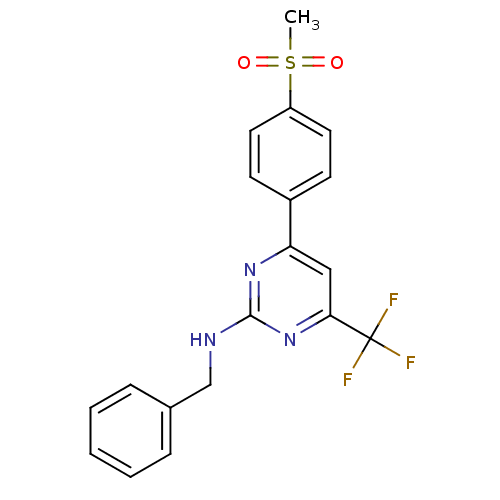 (CHEMBL551829 | N-benzyl-4-(4-(methylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297675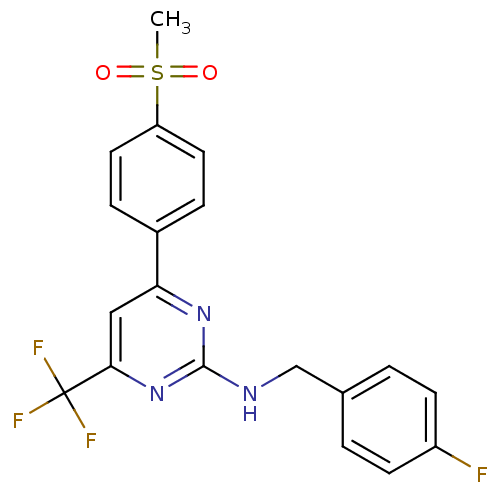 (CHEMBL551148 | N-(4-fluorobenzyl)-4-(4-(methylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297669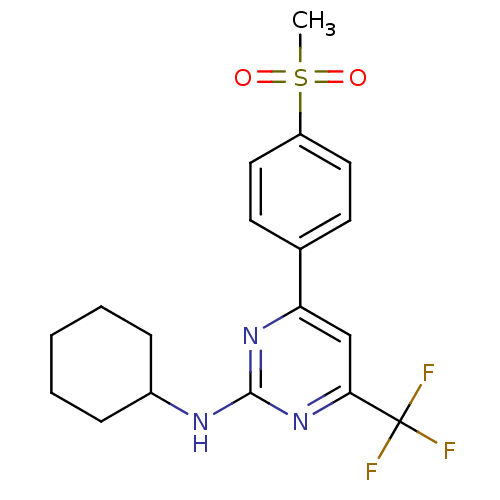 (CHEMBL561891 | N-cyclohexyl-4-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042625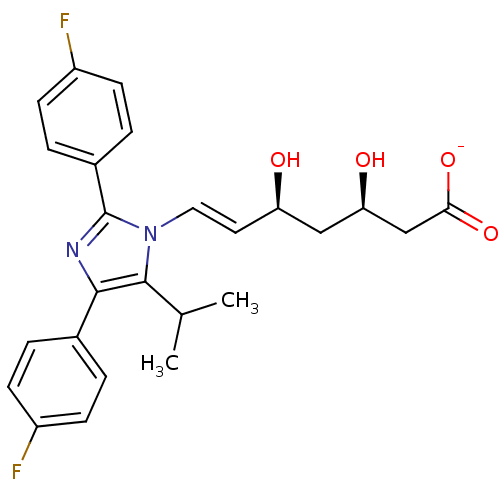 ((E)-(3R,5S)-7-[2,4-Bis-(4-fluoro-phenyl)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042631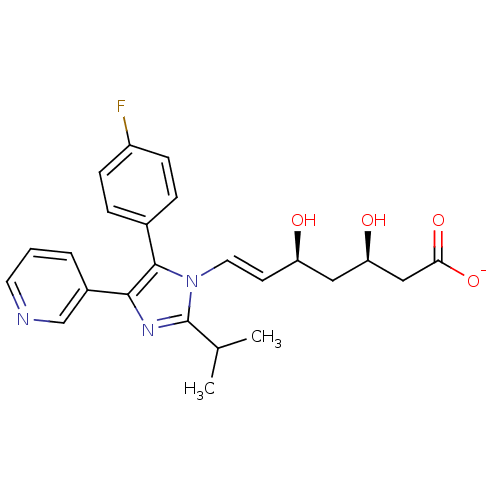 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042607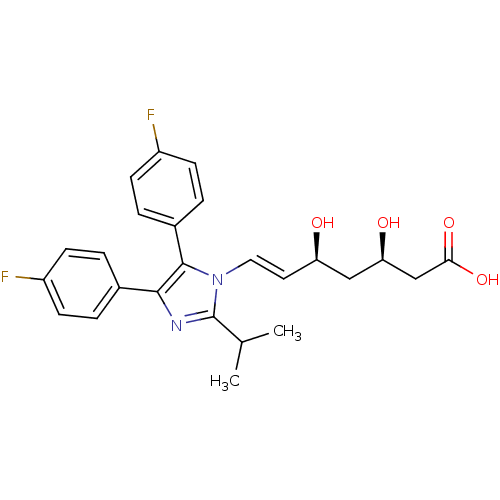 ((E)-(3R,5S)-7-[4,5-Bis-(4-fluoro-phenyl)-2-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297672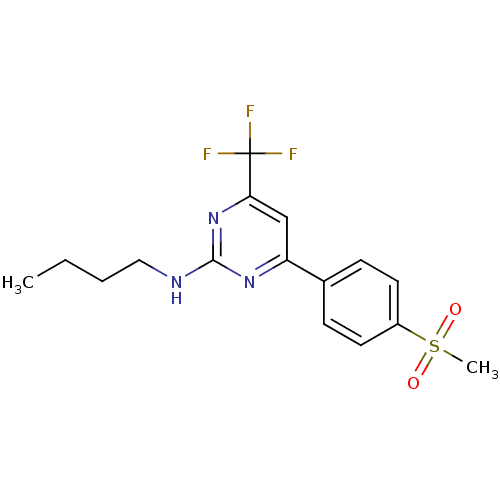 (CHEMBL549393 | N-butyl-4-(4-(methylsulfonyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297671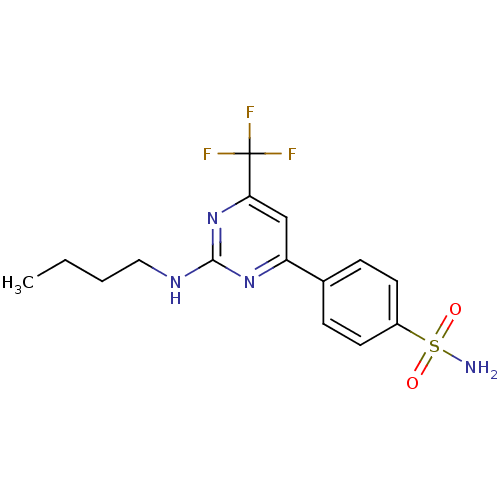 (4-(2-(butylamino)-6-(trifluoromethyl)pyrimidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297670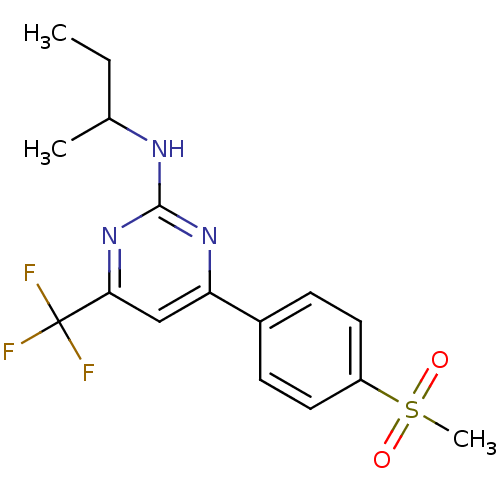 (CHEMBL539663 | GW-637185X | N-sec-butyl-4-(4-(meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281113 (2,6-dihydroxy-7-methylcarbonyloxy-5-[3-(2-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042614 (CHEMBL120932 | Sodium; 7-[4,5-bis-(4-fluoro-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042620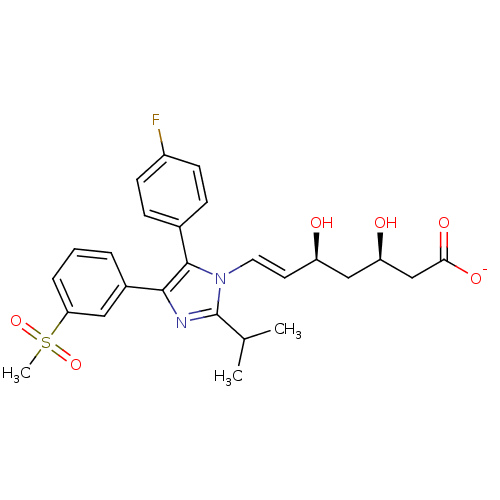 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042615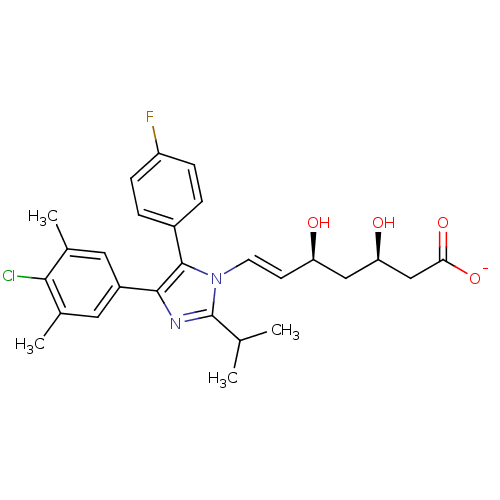 ((E)-(3R,5S)-7-[4-(4-Chloro-3,5-dimethyl-phenyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50281106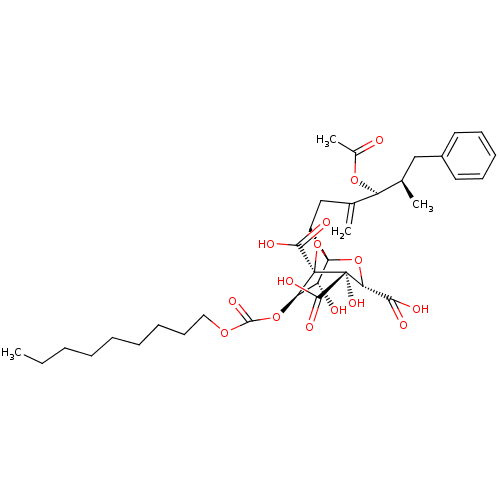 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Candida albicans squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297668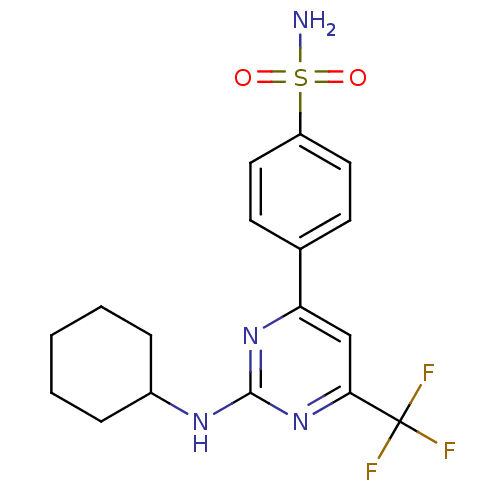 (4-(2-(cyclohexylamino)-6-(trifluoromethyl)pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042629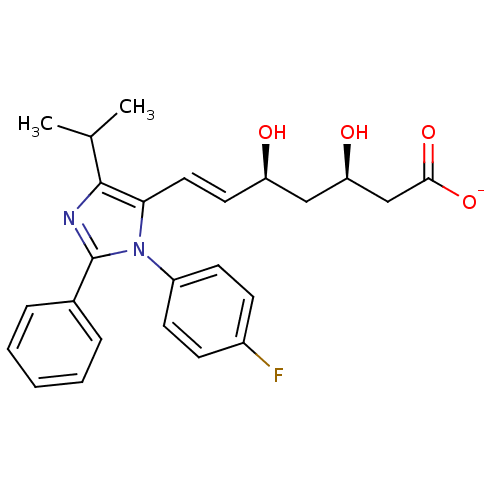 (CHEMBL121309 | Sodium; 7-[3-(4-fluoro-phenyl)-5-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297664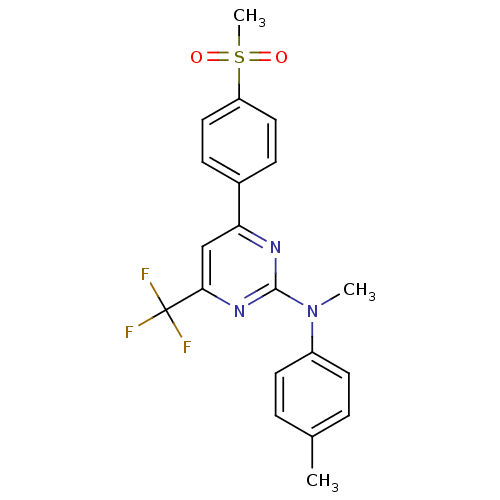 (CHEMBL559613 | N-methyl-4-(4-(methylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281105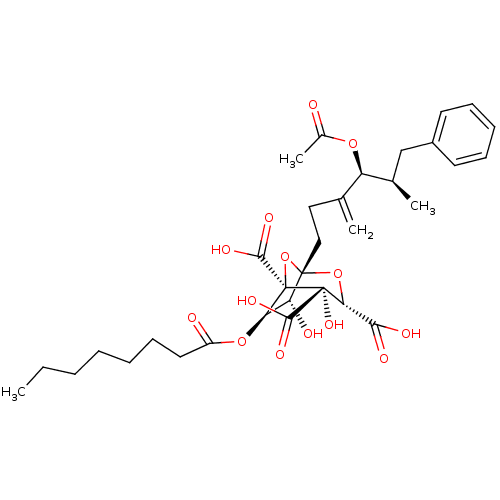 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042622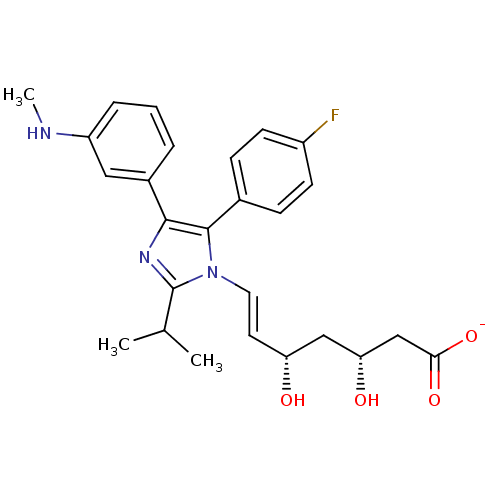 ((E)-(3R,5S)-7-[5-(4-Fluoro-phenyl)-2-isopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042601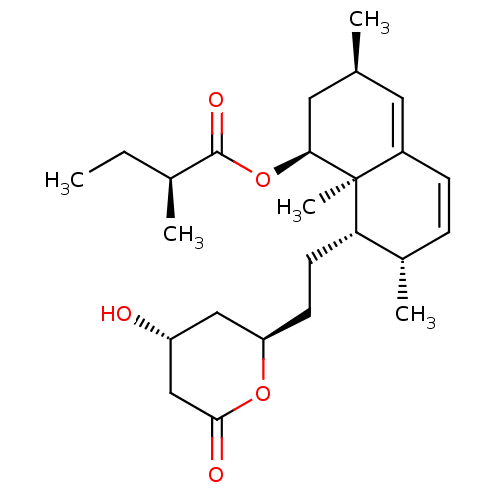 (2-Methyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281109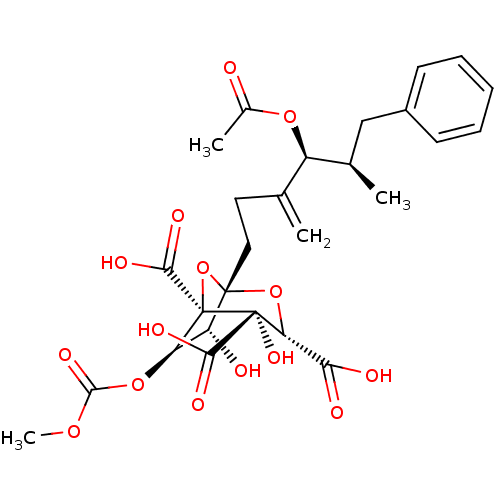 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042612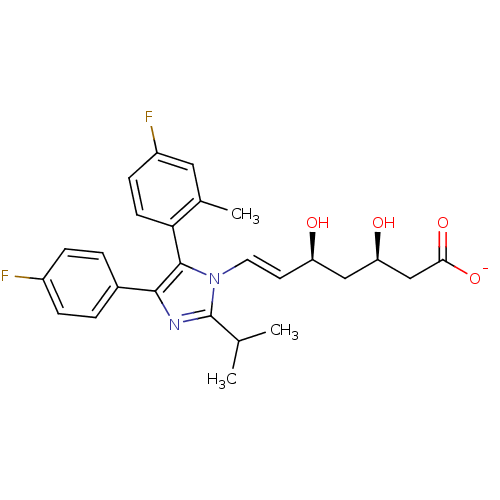 (CHEMBL121610 | Sodium; 7-[5-(4-fluoro-2-methyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50281101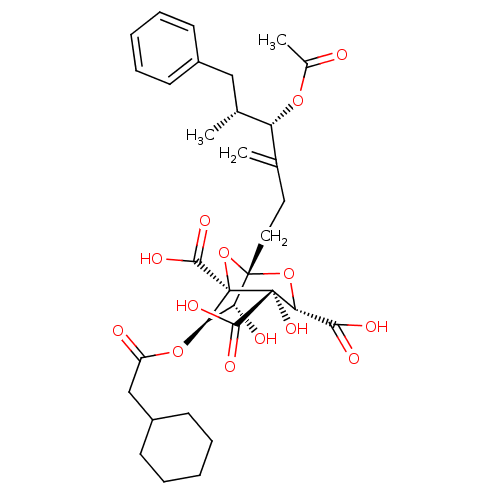 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Candida albicans squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281119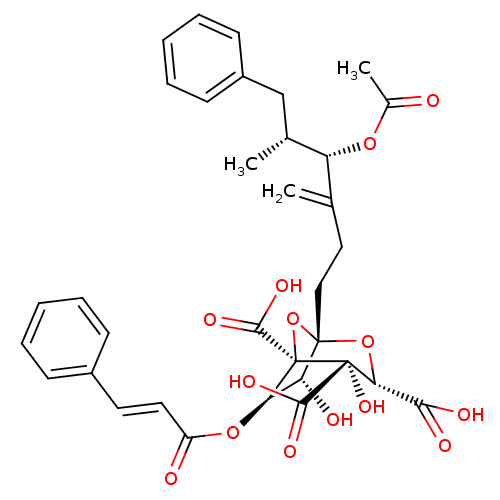 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281102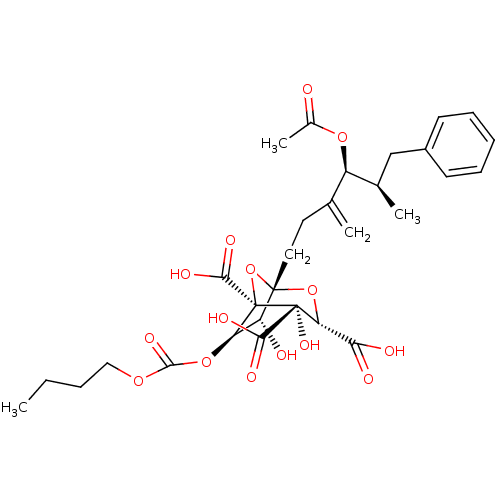 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297673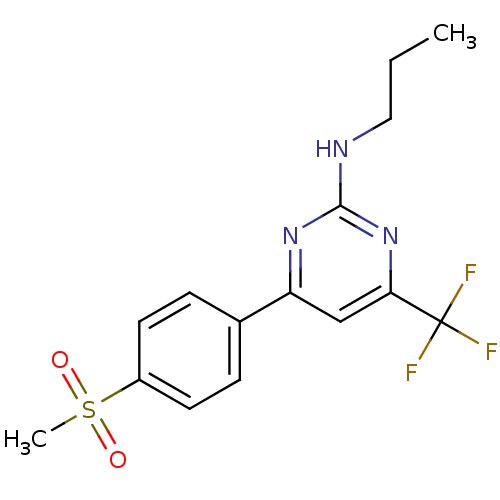 (4-(4-(methylsulfonyl)phenyl)-N-propyl-6-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297665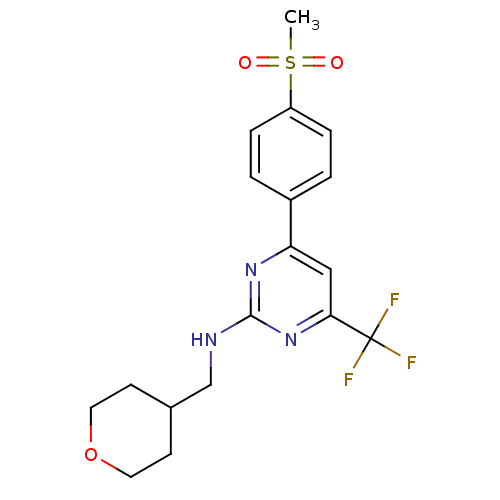 (4-(4-(methylsulfonyl)phenyl)-N-((tetrahydro-2H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042611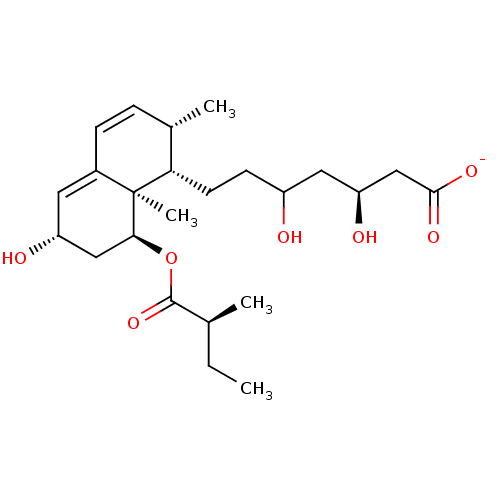 (CHEMBL333003 | Sodium; 3,5-dihydroxy-7-[6-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50051873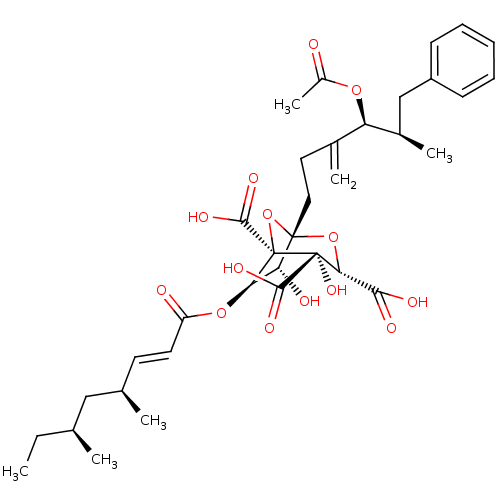 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Candida albicans squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297680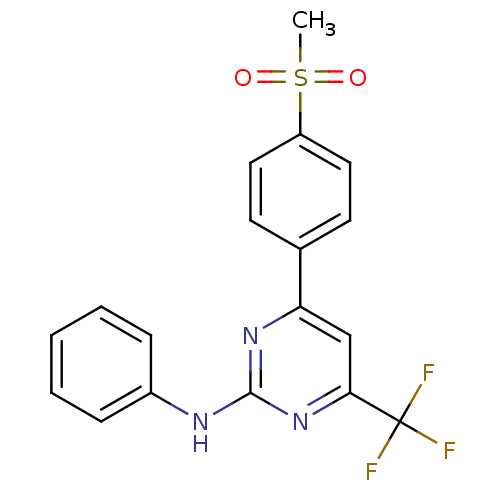 (4-(4-(methylsulfonyl)phenyl)-N-phenyl-6-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051872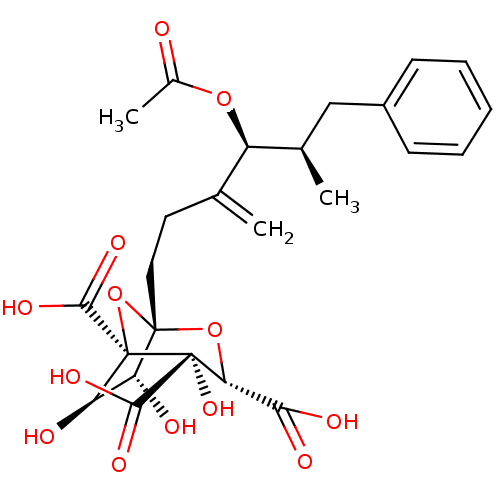 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281122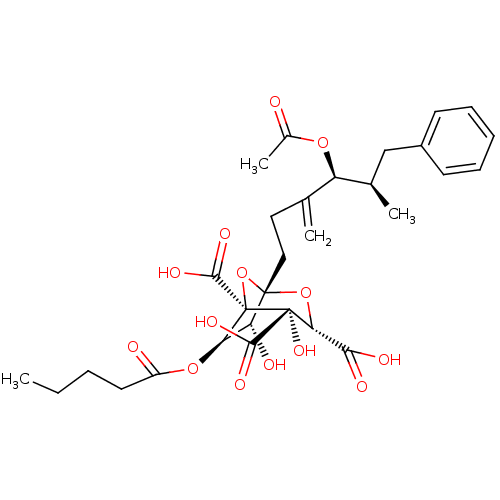 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50042616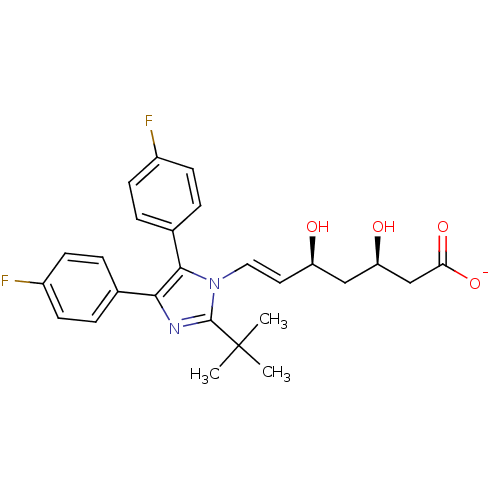 ((E)-(3R,5S)-7-[2-tert-Butyl-4,5-bis-(4-fluoro-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against washed rat liver microsomal HMG-CoA reductase (HMGR) | J Med Chem 36: 3646-57 (1994) BindingDB Entry DOI: 10.7270/Q2X929CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50281116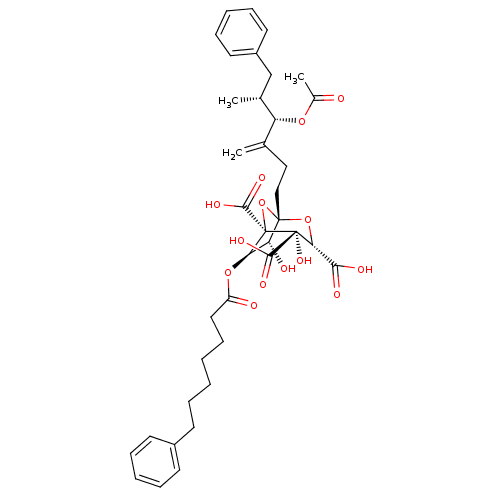 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Candida albicans squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281106 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297678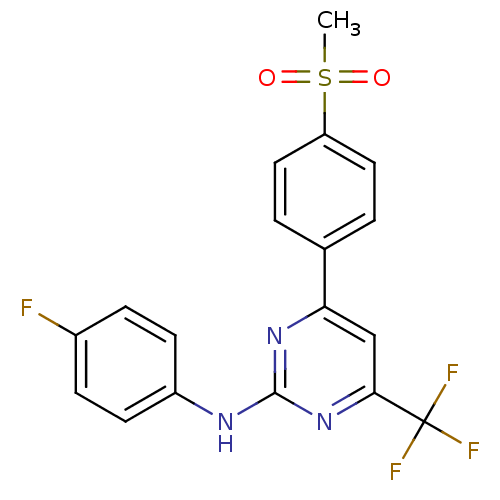 (CHEMBL561086 | N-(4-fluorophenyl)-4-(4-(methylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... | Bioorg Med Chem Lett 19: 4504-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.085 BindingDB Entry DOI: 10.7270/Q20Z7396 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281103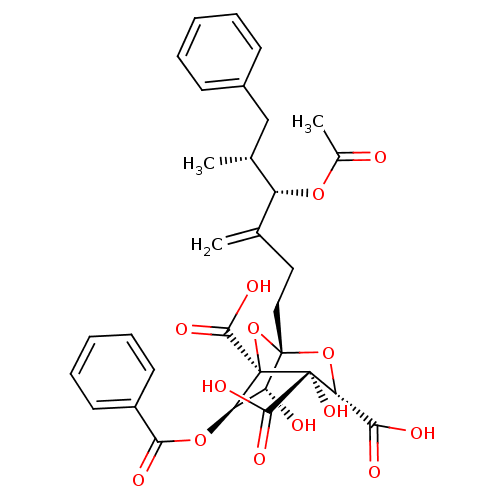 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50281102 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Candida albicans squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50281115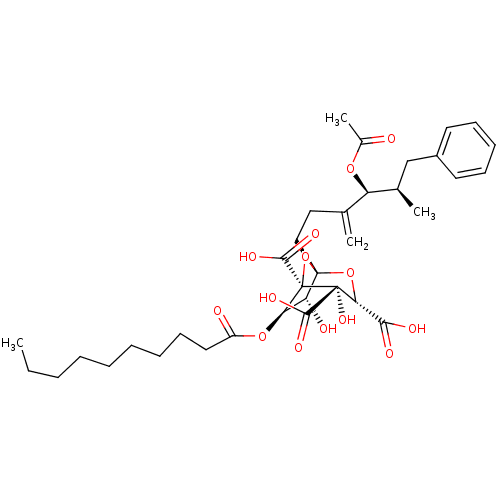 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase(SQS) | Bioorg Med Chem Lett 3: 2605-2610 (1993) Article DOI: 10.1016/S0960-894X(01)80724-1 BindingDB Entry DOI: 10.7270/Q20P0ZZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 205 total ) | Next | Last >> |