

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50546172 (CHEMBL4762397) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00317 BindingDB Entry DOI: 10.7270/Q20K2D40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 8 (Homo sapiens (Human)) | BDBM50586261 (CHEMBL5078689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TLR8 expressed in human PBMC cells assessed as inhibition of ssRNA stimulated TNF level preincubated for 30 mins followe... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00696 BindingDB Entry DOI: 10.7270/Q2W66QPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 8 (Homo sapiens (Human)) | BDBM50586262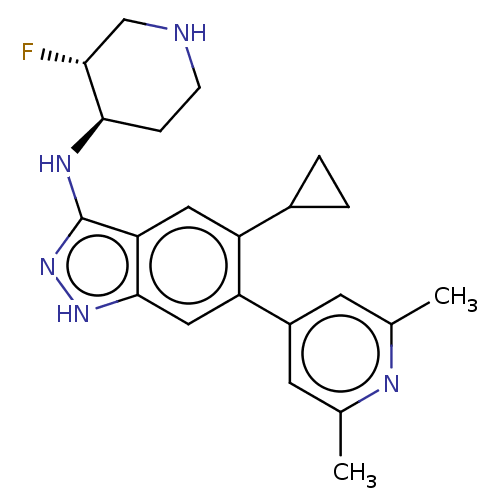 (CHEMBL5091113) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TLR8 expressed in human PBMC cells assessed as inhibition of ssRNA stimulated TNF level preincubated for 30 mins followe... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00696 BindingDB Entry DOI: 10.7270/Q2W66QPP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Toll-like receptor 8 (Homo sapiens (Human)) | BDBM50586259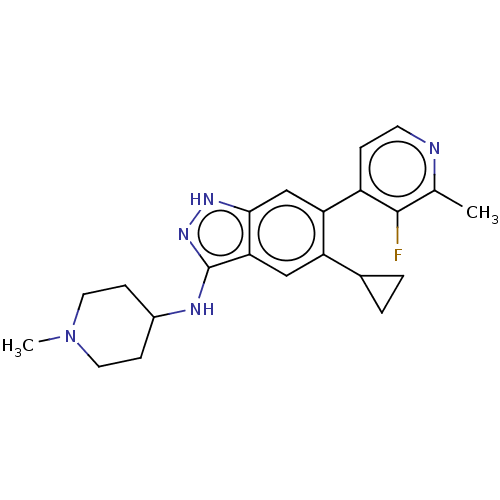 (CHEMBL5090067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TLR8 expressed in human PBMC cells assessed as inhibition of ssRNA stimulated TNF level preincubated for 30 mins followe... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00696 BindingDB Entry DOI: 10.7270/Q2W66QPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM50586260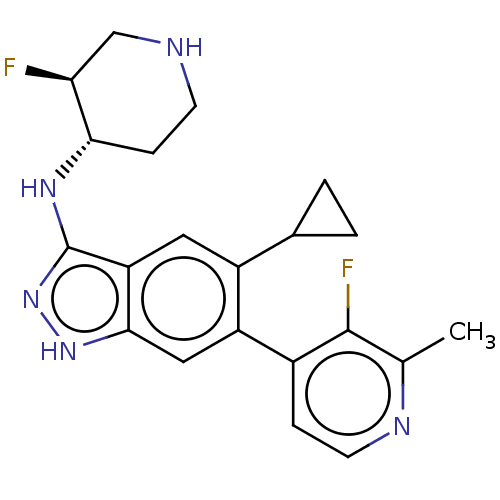 (CHEMBL5079910) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TLR7 expressed in human PBMC cells assessed as inhibition of ssRNA stimulated IFNalpha level preincubated for 30 mins fo... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00696 BindingDB Entry DOI: 10.7270/Q2W66QPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259423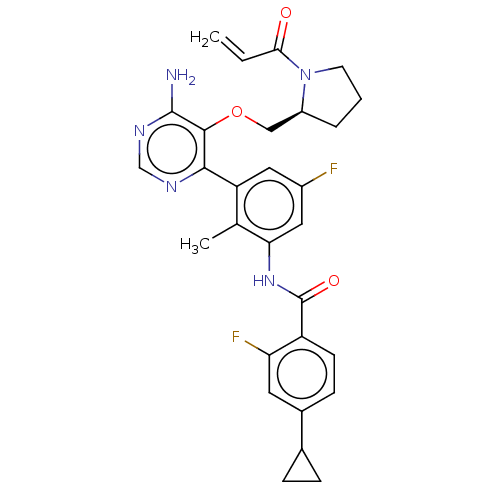 (US10457647, Example 22 | US11180460, Example 22 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q9F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127573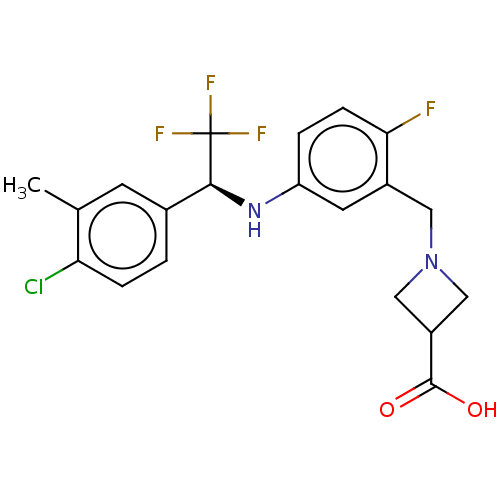 (US8791100, 71) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127572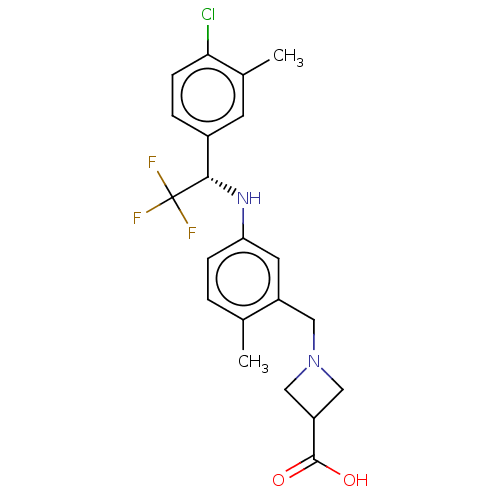 (US8791100, 70) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259423 (US10457647, Example 22 | US11180460, Example 22 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259423 (US10457647, Example 22 | US11180460, Example 22 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US10457647 (2019) BindingDB Entry DOI: 10.7270/Q2KH0QPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127560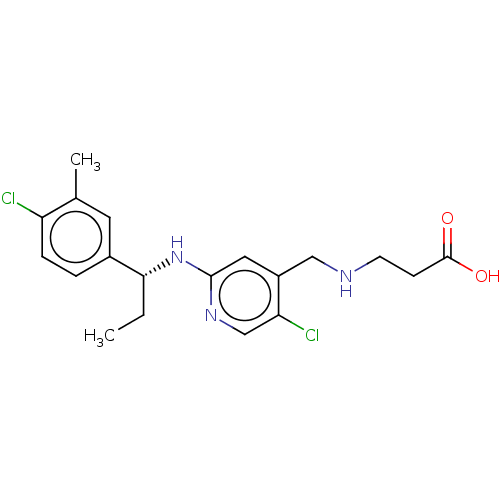 (US8791100, 58) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259423 (US10457647, Example 22 | US11180460, Example 22 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127506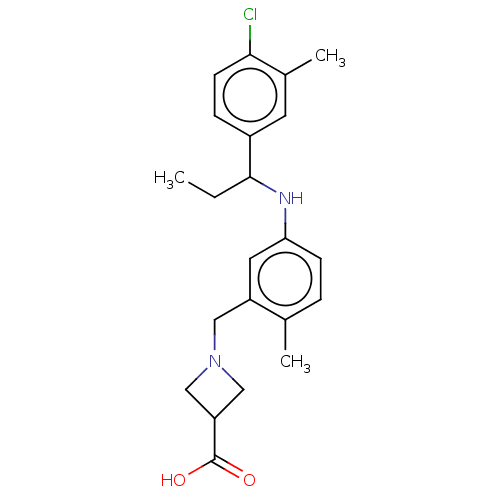 (US8791100, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 8 (Homo sapiens (Human)) | BDBM50586260 (CHEMBL5079910) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TLR8 expressed in human PBMC cells assessed as inhibition of ssRNA stimulated TNF level preincubated for 30 mins followe... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00696 BindingDB Entry DOI: 10.7270/Q2W66QPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127567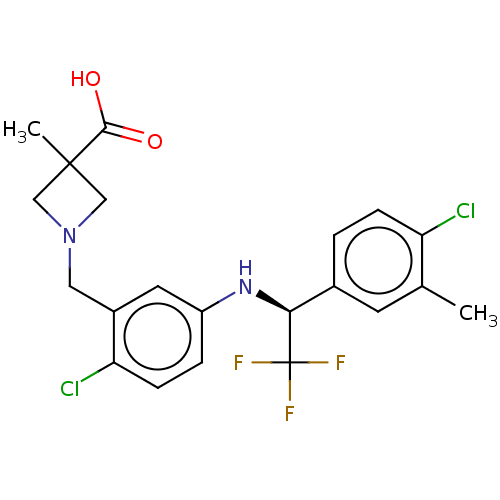 (US8791100, 65) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM50586259 (CHEMBL5090067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TLR7 expressed in human PBMC cells assessed as inhibition of ssRNA stimulated IFNalpha level preincubated for 30 mins fo... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00696 BindingDB Entry DOI: 10.7270/Q2W66QPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259427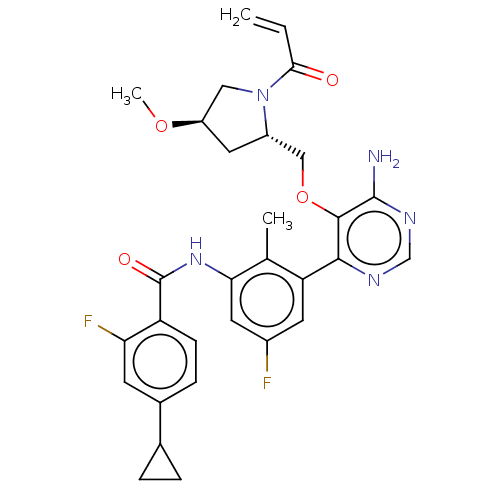 (US10457647, Example 26 | US11180460, Example 26 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US10457647 (2019) BindingDB Entry DOI: 10.7270/Q2KH0QPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127531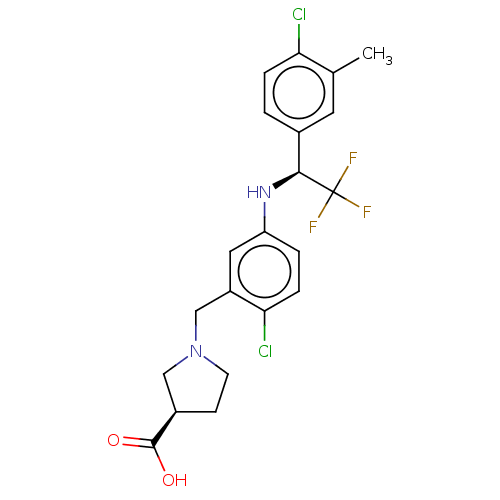 (US8791100, 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259427 (US10457647, Example 26 | US11180460, Example 26 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127511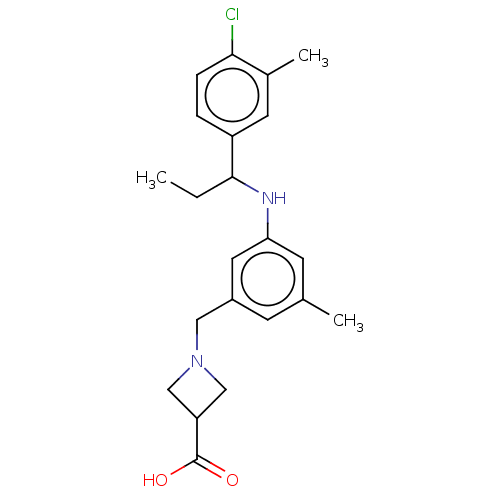 (US8791100, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259427 (US10457647, Example 26 | US11180460, Example 26 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127542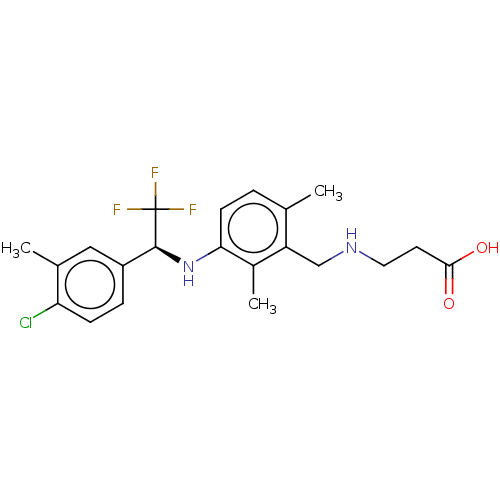 (US8791100, 40) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127586 (US8791100, 84) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259427 (US10457647, Example 26 | US11180460, Example 26 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q9F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127557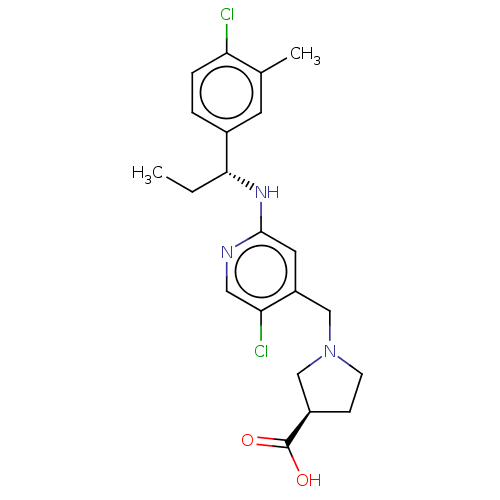 (US8791100, 55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127508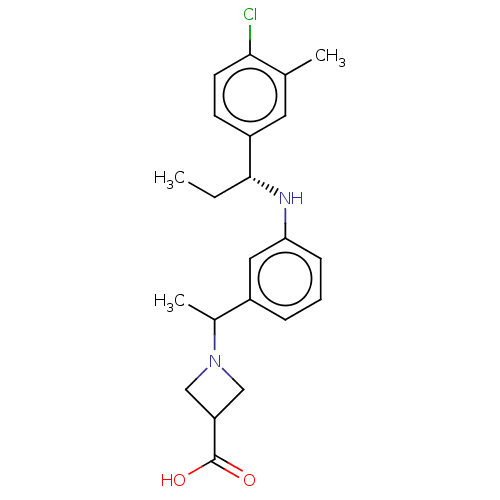 (US8791100, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259425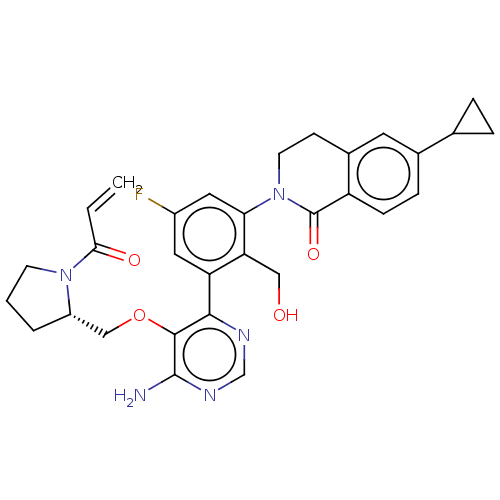 (US10457647, Example 24 | US11180460, Example 24 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q9F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259425 (US10457647, Example 24 | US11180460, Example 24 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259425 (US10457647, Example 24 | US11180460, Example 24 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US10457647 (2019) BindingDB Entry DOI: 10.7270/Q2KH0QPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259425 (US10457647, Example 24 | US11180460, Example 24 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50546180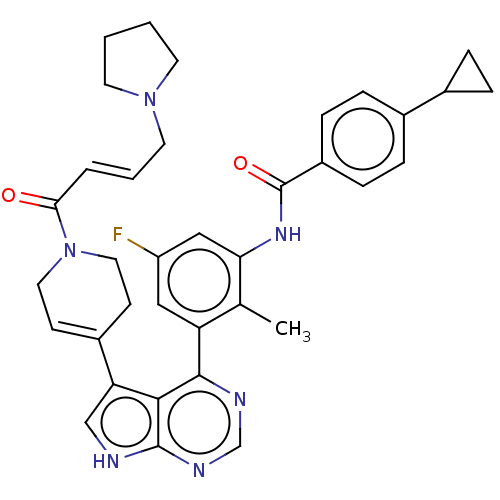 (CHEMBL4749522) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00317 BindingDB Entry DOI: 10.7270/Q20K2D40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127581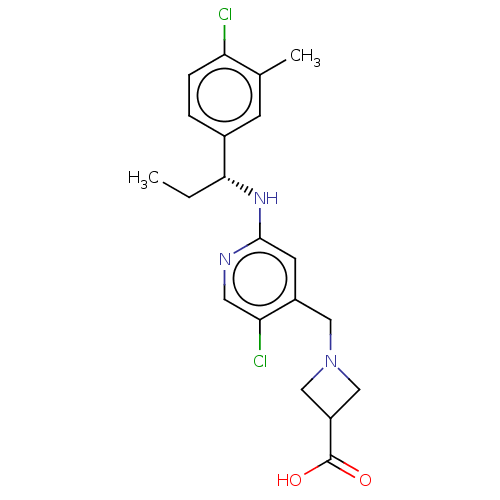 (US8791100, 79) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 8 (Homo sapiens (Human)) | BDBM50586262 (CHEMBL5091113) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TLR8 expressed in human whole blood assessed as inhibition of ssRNA stimulated TNF level preincubated for 30 mins follow... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00696 BindingDB Entry DOI: 10.7270/Q2W66QPP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127535 (US8791100, 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM50586256 (CHEMBL5085458) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TLR7 expressed in human PBMC cells assessed as inhibition of ssRNA stimulated IFNalpha level preincubated for 30 mins fo... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00696 BindingDB Entry DOI: 10.7270/Q2W66QPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127588 (US8791100, 86 | US8791100, 87) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127570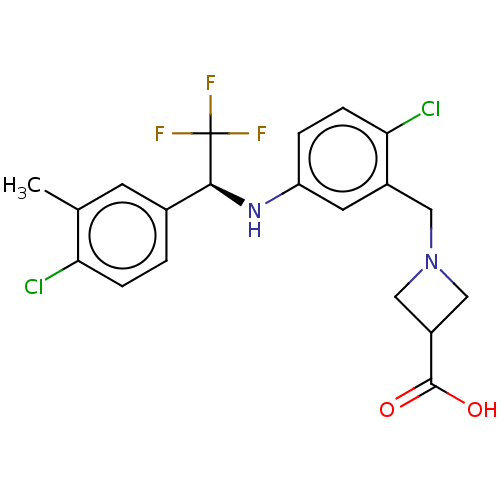 (US8791100, 68) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127536 (US8791100, 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127580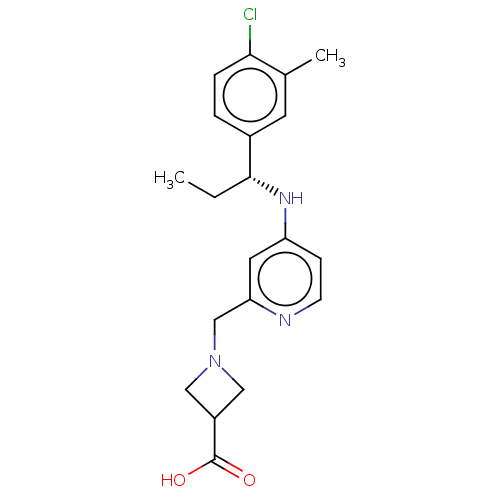 (US8791100, 78) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127571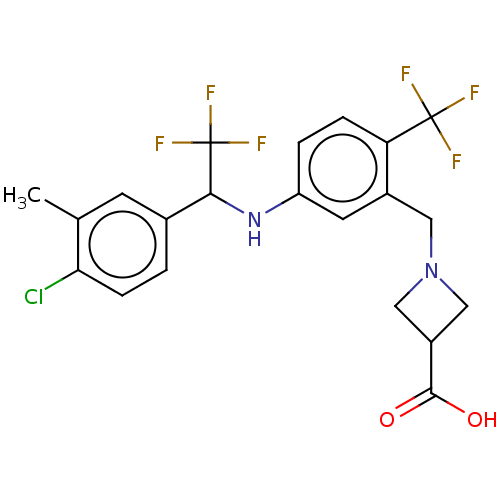 (US8791100, 69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127530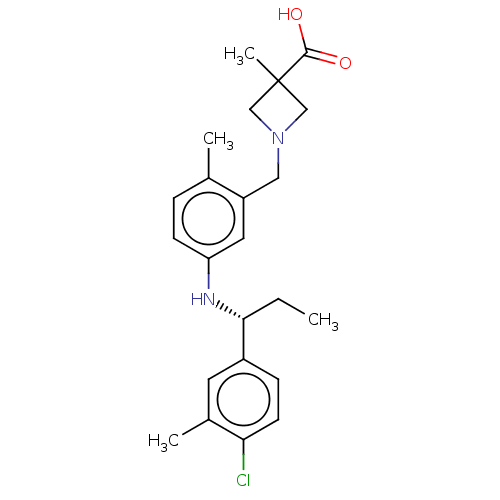 (US8791100, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127569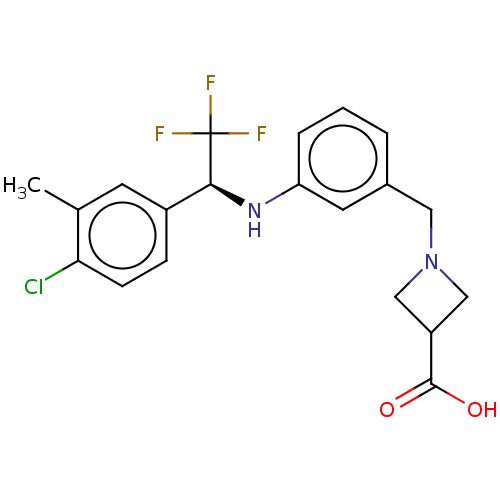 (US8791100, 67) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127534 (US8791100, 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127540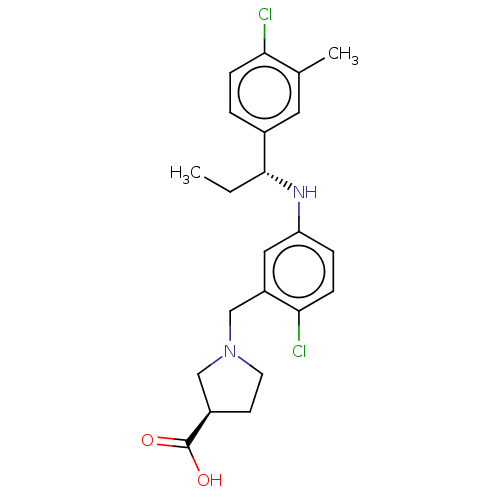 (US8791100, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127541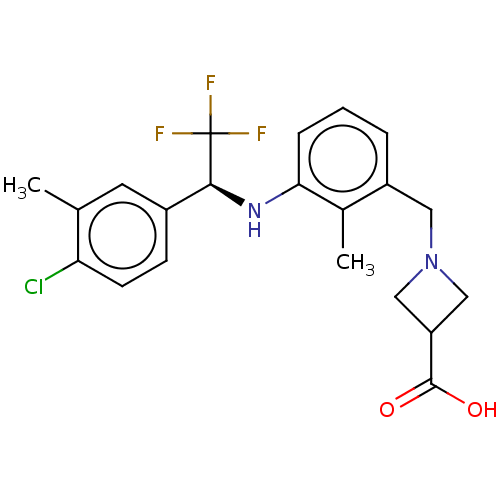 (US8791100, 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127545 (US8791100, 43) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127524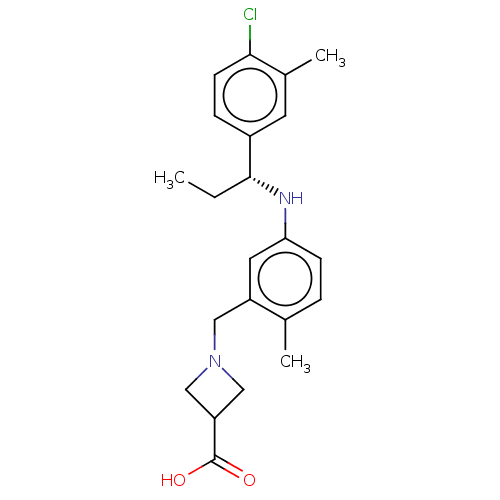 (US8791100, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127587 (US8791100, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127532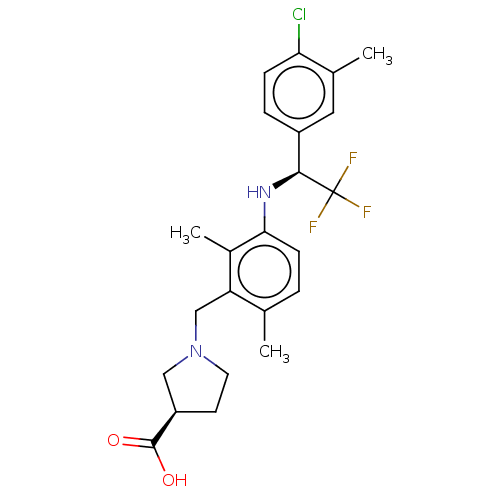 (US8791100, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM127505 (US8791100, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 37 |
Novartis AG US Patent | Assay Description The assay measures intracellular changes of Ca2+ mediated by the synthetic probing agonist 3-{[2-(2-Trifluoromethyl-biphenyl-4-yl)-benzo[b]thiophen-5... | US Patent US8791100 (2014) BindingDB Entry DOI: 10.7270/Q2ZK5FCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 492 total ) | Next | Last >> |