 Found 58 hits with Last Name = 'castro' and Initial = 'd'
Found 58 hits with Last Name = 'castro' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM17051

(BX-795 | BX-795, 3 | N-(3-{[5-iodo-4-({3-[(thiophe...)Show SMILES Ic1cnc(Nc2cccc(NC(=O)N3CCCC3)c2)nc1NCCCNC(=O)c1cccs1 Show InChI InChI=1S/C23H26IN7O2S/c24-18-15-27-22(30-20(18)25-9-5-10-26-21(32)19-8-4-13-34-19)28-16-6-3-7-17(14-16)29-23(33)31-11-1-2-12-31/h3-4,6-8,13-15H,1-2,5,9-12H2,(H,26,32)(H,29,33)(H2,25,27,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cGAS in human THP1 cells assessed as reduction in salmon sperm dsDNA-induced IFN-beta expression preincubated for 1 hr followed by dsDN... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50270528
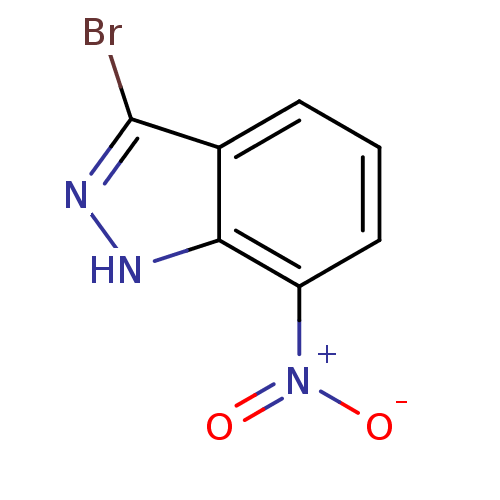
(3-BROMO-7-NITROINDAZOLE | 3-bromo-7-nitro-1H-indaz...)Show InChI InChI=1S/C7H4BrN3O2/c8-7-4-2-1-3-5(11(12)13)6(4)9-10-7/h1-3H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50270528

(3-BROMO-7-NITROINDAZOLE | 3-bromo-7-nitro-1H-indaz...)Show InChI InChI=1S/C7H4BrN3O2/c8-7-4-2-1-3-5(11(12)13)6(4)9-10-7/h1-3H,(H,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS2 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50304142

(2,7-dinitro-2H-indazole | CHEMBL593105)Show InChI InChI=1S/C7H4N4O4/c12-10(13)6-3-1-2-5-4-9(11(14)15)8-7(5)6/h1-4H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209245

(CHEMBL247378)Show InChI InChI=1S/C7H5N3O2/c11-10(12)6-3-1-2-5-4-8-9-7(5)6/h1-4H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Leishmania infantum) | BDBM50363766
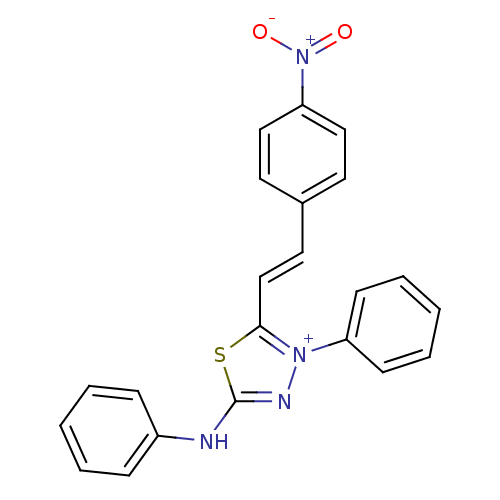
(CHEMBL242165)Show SMILES [O-][N+](=O)c1ccc(\C=C\c2sc(Nc3ccccc3)n[n+]2-c2ccccc2)cc1 Show InChI InChI=1S/C22H17N4O2S/c27-26(28)20-14-11-17(12-15-20)13-16-21-25(19-9-5-2-6-10-19)24-22(29-21)23-18-7-3-1-4-8-18/h1-16H,(H,23,24)/q+1/b16-13+ | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Oswaldo Cruz
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant Leishmania infantum MHOM/MA67ITMAP263 trypanothione reductase assessed as inhibition of NADPH consumption u... |
Bioorg Med Chem 20: 1760-6 (2012)
Article DOI: 10.1016/j.bmc.2012.01.009
BindingDB Entry DOI: 10.7270/Q2Z89CV7 |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250108
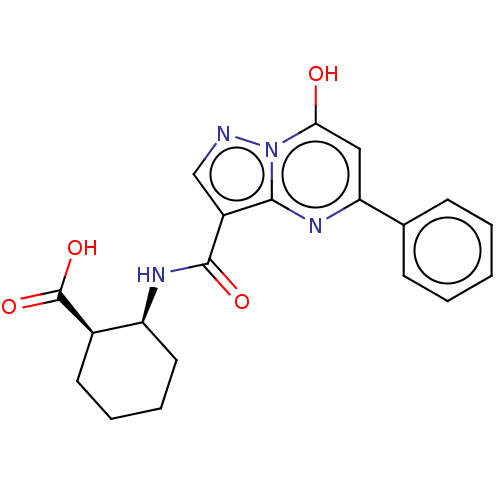
(CHEMBL4096573)Show SMILES OC(=O)[C@@H]1CCCC[C@@H]1NC(=O)c1cnn2c(O)cc(nc12)-c1ccccc1 |r| Show InChI InChI=1S/C20H20N4O4/c25-17-10-16(12-6-2-1-3-7-12)22-18-14(11-21-24(17)18)19(26)23-15-9-5-4-8-13(15)20(27)28/h1-3,6-7,10-11,13,15,25H,4-5,8-9H2,(H,23,26)(H,27,28)/t13-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209241

(4-bromo-1H-indazole | CHEMBL246393)Show InChI InChI=1S/C7H5BrN2/c8-6-2-1-3-7-5(6)4-9-10-7/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50304143
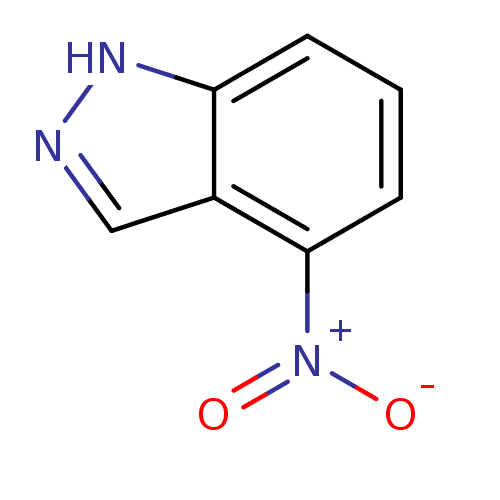
(4-nitro-1H-indazole | CHEMBL393456)Show InChI InChI=1S/C7H5N3O2/c11-10(12)7-3-1-2-6-5(7)4-8-9-6/h1-4H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50304142

(2,7-dinitro-2H-indazole | CHEMBL593105)Show InChI InChI=1S/C7H4N4O4/c12-10(13)6-3-1-2-5-4-9(11(14)15)8-7(5)6/h1-4H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS2 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209244
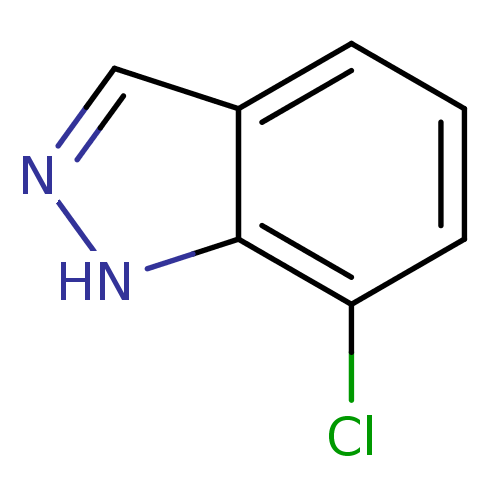
(7-chloro-1H-indazole | CHEMBL247367)Show InChI InChI=1S/C7H5ClN2/c8-6-3-1-2-5-4-9-10-7(5)6/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209237
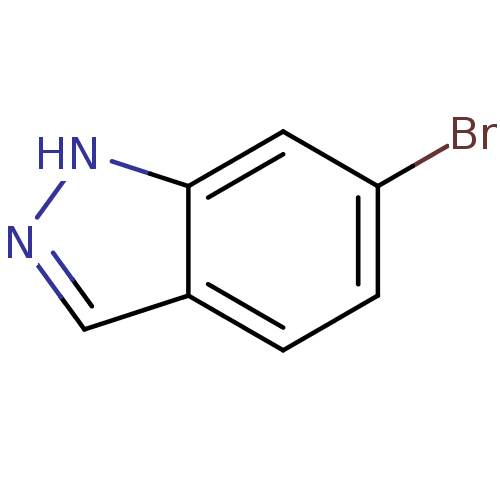
(6-bromo-1H-indazole | CHEMBL247365)Show InChI InChI=1S/C7H5BrN2/c8-6-2-1-5-4-9-10-7(5)3-6/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250108

(CHEMBL4096573)Show SMILES OC(=O)[C@@H]1CCCC[C@@H]1NC(=O)c1cnn2c(O)cc(nc12)-c1ccccc1 |r| Show InChI InChI=1S/C20H20N4O4/c25-17-10-16(12-6-2-1-3-7-12)22-18-14(11-21-24(17)18)19(26)23-15-9-5-4-8-13(15)20(27)28/h1-3,6-7,10-11,13,15,25H,4-5,8-9H2,(H,23,26)(H,27,28)/t13-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50209245

(CHEMBL247378)Show InChI InChI=1S/C7H5N3O2/c11-10(12)6-3-1-2-5-4-8-9-7(5)6/h1-4H,(H,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS2 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250111

(CHEMBL4084664)Show SMILES C[C@@H](CO)NC(=O)c1cnn2c(O)cc(nc12)-c1ccccc1 |r| Show InChI InChI=1S/C16H16N4O3/c1-10(9-21)18-16(23)12-8-17-20-14(22)7-13(19-15(12)20)11-5-3-2-4-6-11/h2-8,10,21-22H,9H2,1H3,(H,18,23)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250109

(CHEMBL4062994)Show InChI InChI=1S/C15H12N4O4/c20-12-6-11(9-4-2-1-3-5-9)18-14-10(7-17-19(12)14)15(23)16-8-13(21)22/h1-7,20H,8H2,(H,16,23)(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209239
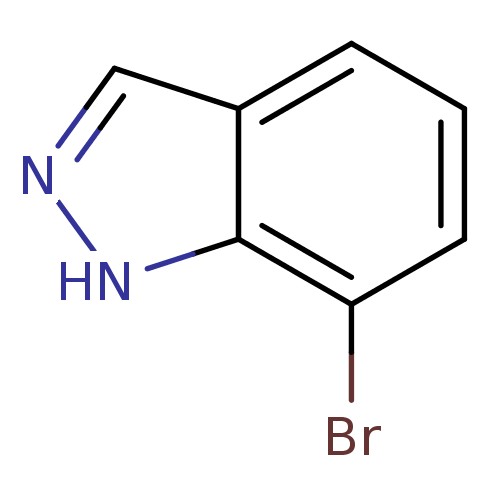
(7-bromo-1H-indazole | CHEMBL439566)Show InChI InChI=1S/C7H5BrN2/c8-6-3-1-2-5-4-9-10-7(5)6/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250106
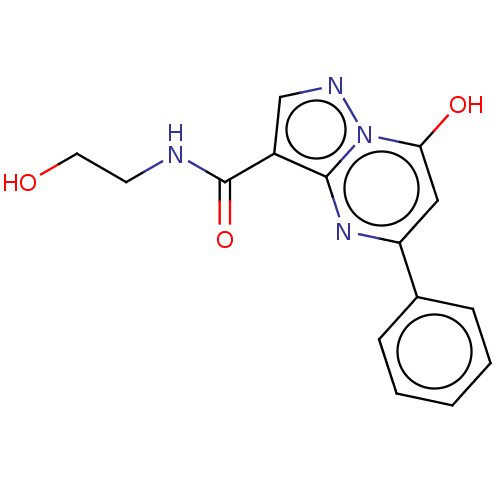
(CHEMBL4103560)Show InChI InChI=1S/C15H14N4O3/c20-7-6-16-15(22)11-9-17-19-13(21)8-12(18-14(11)19)10-4-2-1-3-5-10/h1-5,8-9,20-21H,6-7H2,(H,16,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209235
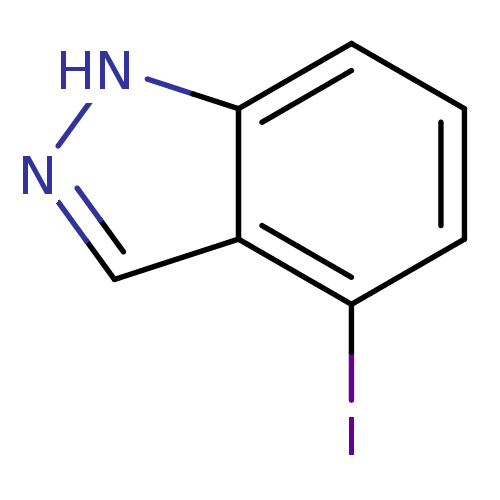
(4-iodo-1H-indazole | CHEMBL246534)Show InChI InChI=1S/C7H5IN2/c8-6-2-1-3-7-5(6)4-9-10-7/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250111

(CHEMBL4084664)Show SMILES C[C@@H](CO)NC(=O)c1cnn2c(O)cc(nc12)-c1ccccc1 |r| Show InChI InChI=1S/C16H16N4O3/c1-10(9-21)18-16(23)12-8-17-20-14(22)7-13(19-15(12)20)11-5-3-2-4-6-11/h2-8,10,21-22H,9H2,1H3,(H,18,23)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250106

(CHEMBL4103560)Show InChI InChI=1S/C15H14N4O3/c20-7-6-16-15(22)11-9-17-19-13(21)8-12(18-14(11)19)10-4-2-1-3-5-10/h1-5,8-9,20-21H,6-7H2,(H,16,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250109

(CHEMBL4062994)Show InChI InChI=1S/C15H12N4O4/c20-12-6-11(9-4-2-1-3-5-9)18-14-10(7-17-19(12)14)15(23)16-8-13(21)22/h1-7,20H,8H2,(H,16,23)(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50365106
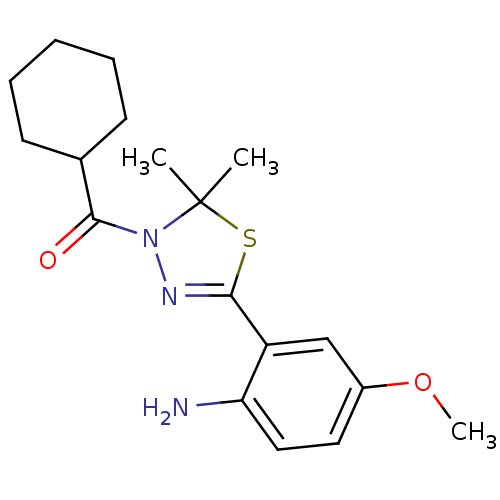
(CHEMBL1951503)Show SMILES COc1ccc(N)c(c1)C1=NN(C(=O)C2CCCCC2)C(C)(C)S1 |t:10| Show InChI InChI=1S/C18H25N3O2S/c1-18(2)21(17(22)12-7-5-4-6-8-12)20-16(24-18)14-11-13(23-3)9-10-15(14)19/h9-12H,4-8,19H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of iNOS assessed as conversion of L-[3H]arginine to L-[3H]-citrulline after 30 mins by scintillation counting |
Eur J Med Chem 50: 129-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.047
BindingDB Entry DOI: 10.7270/Q2V40VP2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209236
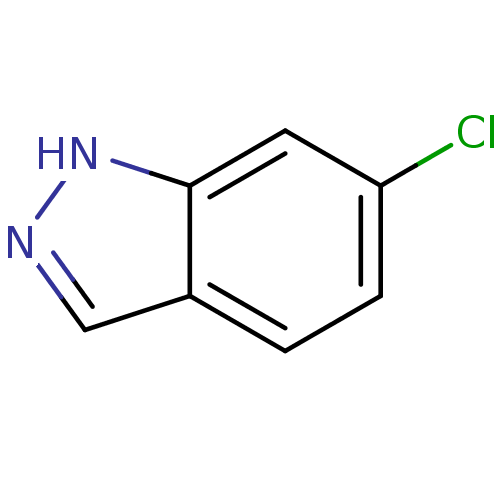
(6-Chloro-1H-indazole | CHEMBL392184)Show InChI InChI=1S/C7H5ClN2/c8-6-2-1-5-4-9-10-7(5)3-6/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50365104
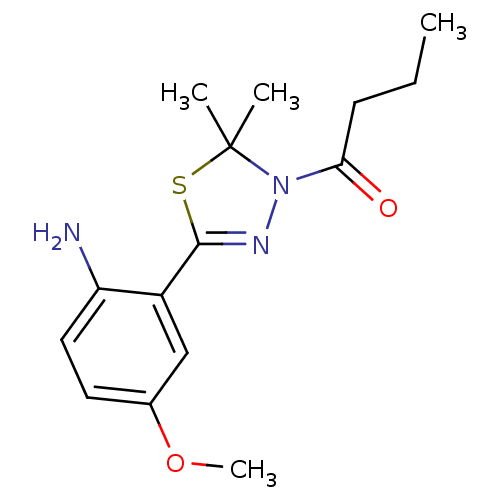
(CHEMBL1951498)Show InChI InChI=1S/C15H21N3O2S/c1-5-6-13(19)18-15(2,3)21-14(17-18)11-9-10(20-4)7-8-12(11)16/h7-9H,5-6,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of iNOS assessed as conversion of L-[3H]arginine to L-[3H]-citrulline after 30 mins by scintillation counting |
Eur J Med Chem 50: 129-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.047
BindingDB Entry DOI: 10.7270/Q2V40VP2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50284964
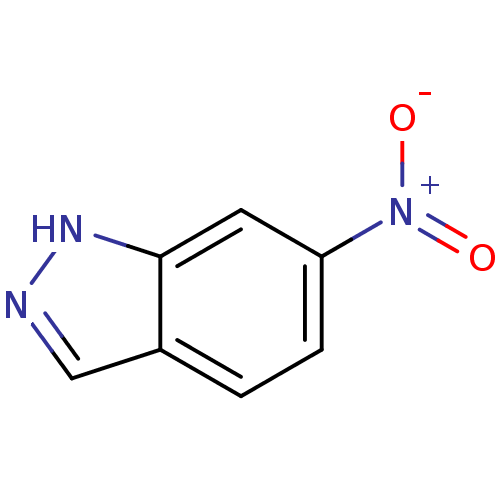
(6-NITROINDAZOLE | 6-Nitro-1H-indazole | CHEMBL5427...)Show InChI InChI=1S/C7H5N3O2/c11-10(12)6-2-1-5-4-8-9-7(5)3-6/h1-4H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50365107
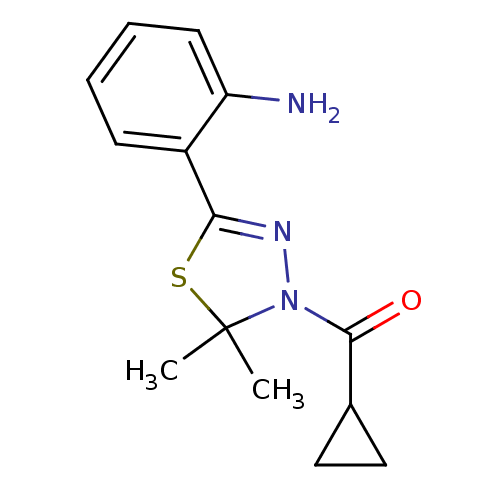
(CHEMBL1951517)Show InChI InChI=1S/C14H17N3OS/c1-14(2)17(13(18)9-7-8-9)16-12(19-14)10-5-3-4-6-11(10)15/h3-6,9H,7-8,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of iNOS assessed as conversion of L-[3H]arginine to L-[3H]-citrulline after 30 mins by scintillation counting |
Eur J Med Chem 50: 129-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.047
BindingDB Entry DOI: 10.7270/Q2V40VP2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209243
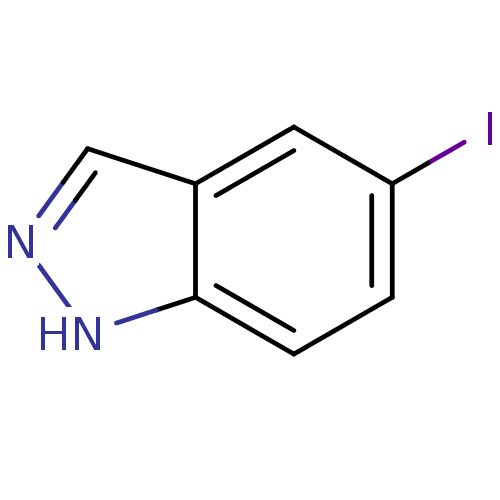
(5-iodo-1H-indazole | CHEMBL391348)Show InChI InChI=1S/C7H5IN2/c8-6-1-2-7-5(3-6)4-9-10-7/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50108560

(2-Acetylamino-4-(2-amino-5-methoxy-phenyl)-4-oxo-b...)Show InChI InChI=1S/C13H16N2O5/c1-7(16)15-11(13(18)19)6-12(17)9-5-8(20-2)3-4-10(9)14/h3-5,11H,6,14H2,1-2H3,(H,15,16)(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against Neuronal nitric oxide synthase (nNOS) activity |
J Med Chem 45: 263-74 (2002)
BindingDB Entry DOI: 10.7270/Q2474966 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50365105
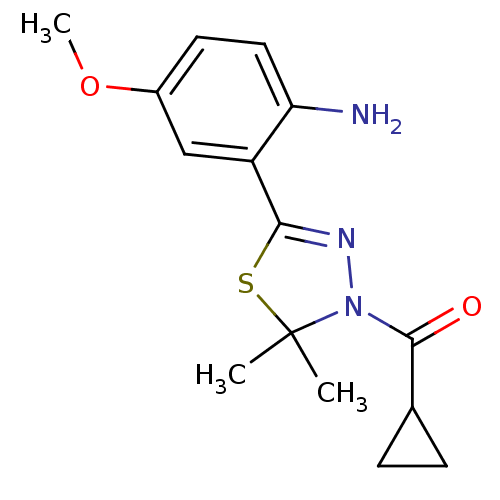
(CHEMBL1951500)Show SMILES COc1ccc(N)c(c1)C1=NN(C(=O)C2CC2)C(C)(C)S1 |t:10| Show InChI InChI=1S/C15H19N3O2S/c1-15(2)18(14(19)9-4-5-9)17-13(21-15)11-8-10(20-3)6-7-12(11)16/h6-9H,4-5,16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of iNOS assessed as conversion of L-[3H]arginine to L-[3H]-citrulline after 30 mins by scintillation counting |
Eur J Med Chem 50: 129-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.047
BindingDB Entry DOI: 10.7270/Q2V40VP2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209234
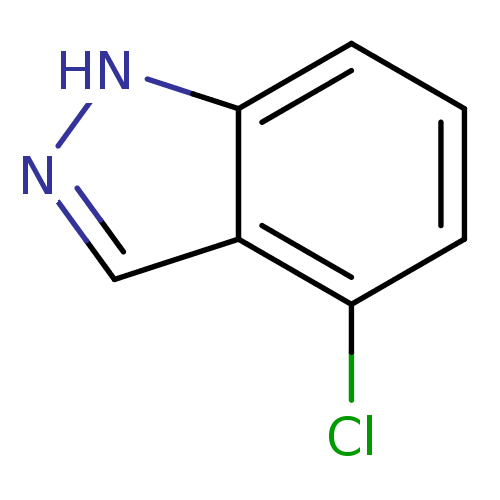
(4-chloro-1H-indazole | CHEMBL246533)Show InChI InChI=1S/C7H5ClN2/c8-6-2-1-3-7-5(6)4-9-10-7/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50304144
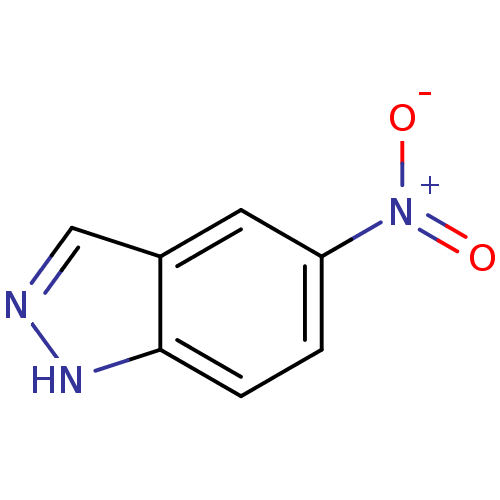
(5-NITROINDAZOLE | 5-Nitro-1H-indazole | CHEMBL1653...)Show InChI InChI=1S/C7H5N3O2/c11-10(12)6-1-2-7-5(3-6)4-8-9-7/h1-4H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209242
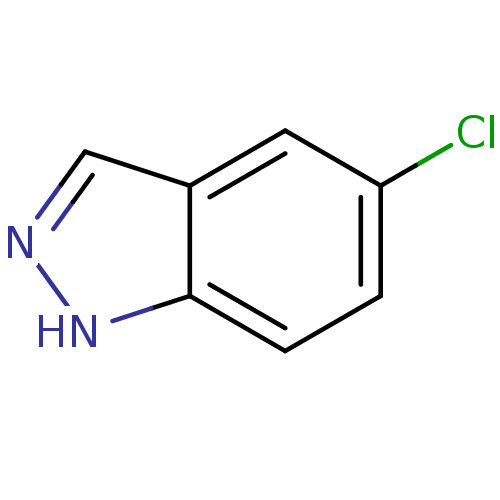
(5-chloro-1H-indazole | CHEMBL246746)Show InChI InChI=1S/C7H5ClN2/c8-6-1-2-7-5(3-6)4-9-10-7/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250107

(CHEMBL4085628)Show InChI InChI=1S/C14H12N4O2/c1-15-14(20)10-8-16-18-12(19)7-11(17-13(10)18)9-5-3-2-4-6-9/h2-8,19H,1H3,(H,15,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50099398
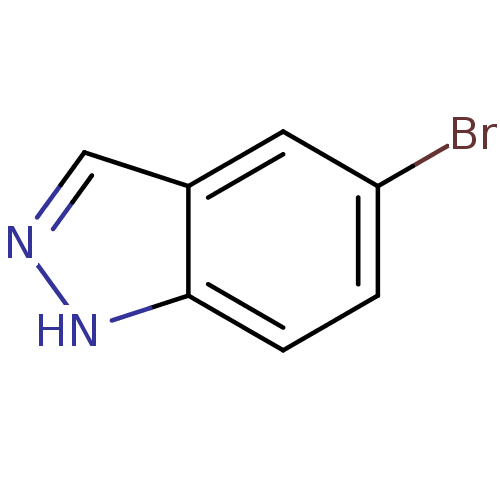
(5-Bromo-1H-indazole | CHEMBL16425 | cid_761929)Show InChI InChI=1S/C7H5BrN2/c8-6-1-2-7-5(3-6)4-9-10-7/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250110
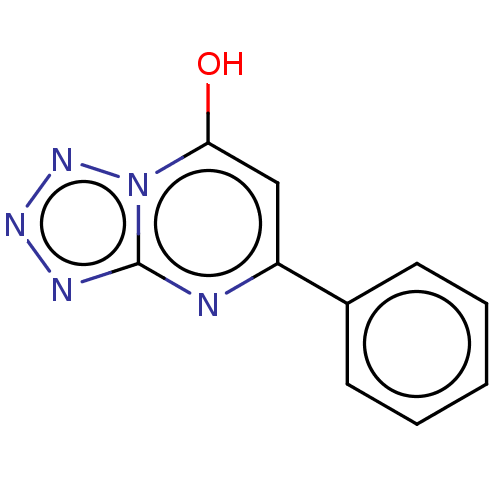
(CHEMBL4065757)Show InChI InChI=1S/C10H7N5O/c16-9-6-8(7-4-2-1-3-5-7)11-10-12-13-14-15(9)10/h1-6,16H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50304147
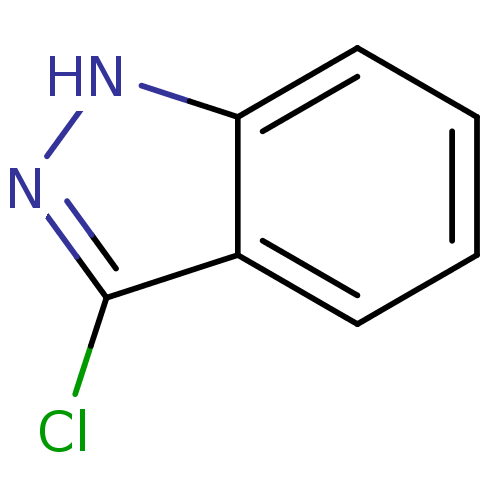
(3-chloro-1H-indazole | CHEMBL596385)Show InChI InChI=1S/C7H5ClN2/c8-7-5-3-1-2-4-6(5)9-10-7/h1-4H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of recombinant NOS1 assessed as citrulline formation |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50271275
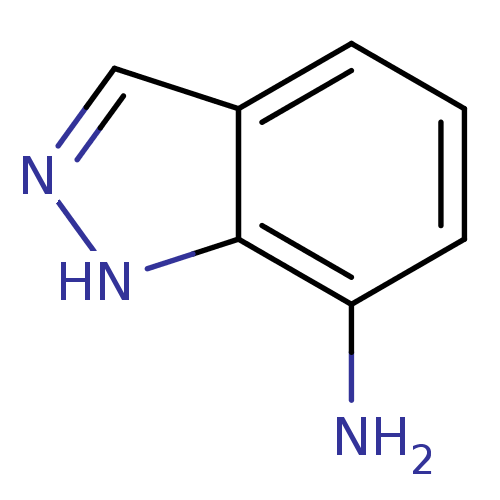
(1H-Indazol-7-amine | 1H-indazol-7-amine, 1 | CHEMB...)Show InChI InChI=1S/C7H7N3/c8-6-3-1-2-5-4-9-10-7(5)6/h1-4H,8H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of recombinant NOS1 assessed as citrulline formation |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Cyclic GMP-AMP synthase
(Homo sapiens) | BDBM50250107

(CHEMBL4085628)Show InChI InChI=1S/C14H12N4O2/c1-15-14(20)10-8-16-18-12(19)7-11(17-13(10)18)9-5-3-2-4-6-9/h2-8,19H,1H3,(H,15,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... |
PLoS ONE 12: (2017)
Article DOI: 10.1371/journal.pone.0184843
BindingDB Entry DOI: 10.7270/Q2WQ067J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50304145
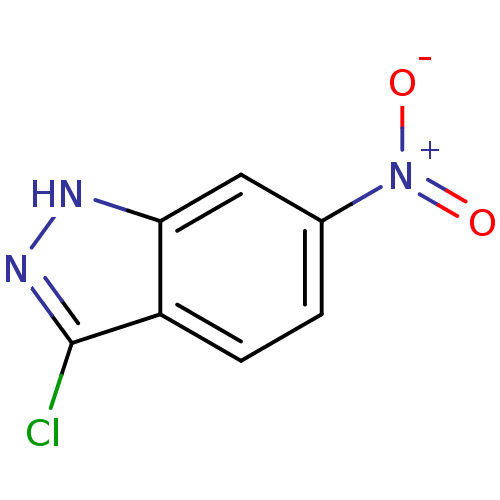
(3-chloro-6-nitro-1H-indazole | CHEMBL596229)Show InChI InChI=1S/C7H4ClN3O2/c8-7-5-2-1-4(11(12)13)3-6(5)9-10-7/h1-3H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS1 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM24627
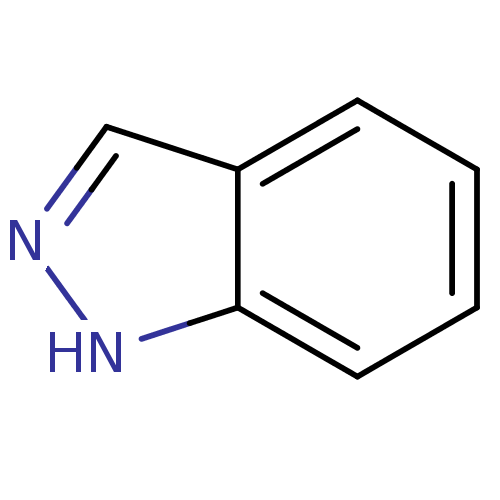
(1H-indazole | Indazole, 5 | Indazole, 6)Show InChI InChI=1S/C7H6N2/c1-2-4-7-6(3-1)5-8-9-7/h1-5H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of recombinant NOS1 assessed as citrulline formation |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50304150

(3-bromo-1,5-dinitro-1H-indazole | CHEMBL596341)Show SMILES [O-][N+](=O)c1ccc2n(nc(Br)c2c1)[N+]([O-])=O Show InChI InChI=1S/C7H3BrN4O4/c8-7-5-3-4(11(13)14)1-2-6(5)10(9-7)12(15)16/h1-3H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of recombinant NOS1 assessed as citrulline formation |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50304146
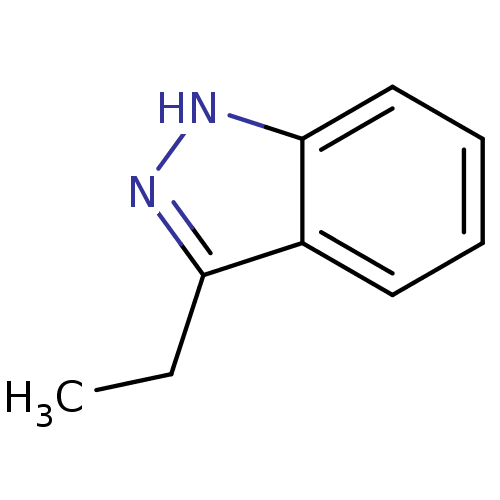
(3-ethyl-1H-indazole | CHEMBL595903)Show InChI InChI=1S/C9H10N2/c1-2-8-7-5-3-4-6-9(7)11-10-8/h3-6H,2H2,1H3,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of recombinant NOS1 assessed as citrulline formation |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50304151
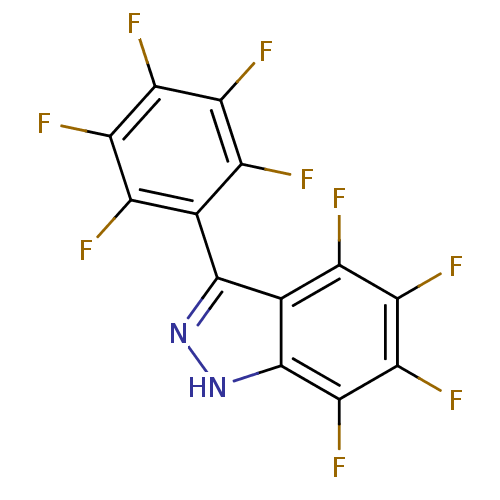
(4,5,6,7-tetrafluoro-3-perfluorophenyl-1H-indazole ...)Show SMILES Fc1c(F)c(F)c(-c2n[nH]c3c(F)c(F)c(F)c(F)c23)c(F)c1F |(10.47,-19.5,;9.99,-20.96,;11.03,-22.11,;12.53,-21.79,;10.55,-23.57,;11.58,-24.72,;9.04,-23.88,;8.57,-25.35,;9.47,-26.59,;8.57,-27.84,;7.1,-27.36,;5.77,-28.14,;5.78,-29.68,;4.43,-27.37,;3.1,-28.14,;4.43,-25.83,;3.1,-25.06,;5.77,-25.06,;5.77,-23.52,;7.1,-25.82,;8.01,-22.75,;6.67,-21.98,;8.48,-21.29,;7.45,-20.15,)| Show InChI InChI=1S/C13HF9N2/c14-3-1(4(15)7(18)9(20)6(3)17)12-2-5(16)8(19)10(21)11(22)13(2)24-23-12/h(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of NOS2 |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50492294
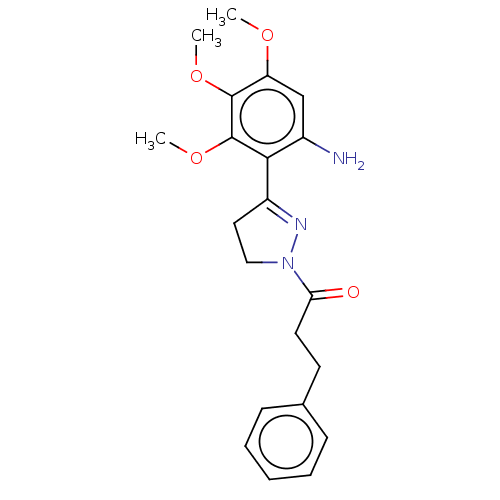
(CHEMBL2397627)Show SMILES COc1cc(N)c(C2=NN(CC2)C(=O)CCc2ccccc2)c(OC)c1OC |t:7| Show InChI InChI=1S/C21H25N3O4/c1-26-17-13-15(22)19(21(28-3)20(17)27-2)16-11-12-24(23-16)18(25)10-9-14-7-5-4-6-8-14/h4-8,13H,9-12,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of recombinant nNOS (unknown origin) assessed as conversion of L-[3H]-arginine to L-[3H]-citrulline after 30 mins |
Bioorg Med Chem 21: 4132-42 (2013)
Article DOI: 10.1016/j.bmc.2013.05.016
BindingDB Entry DOI: 10.7270/Q2CV4MPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50492295
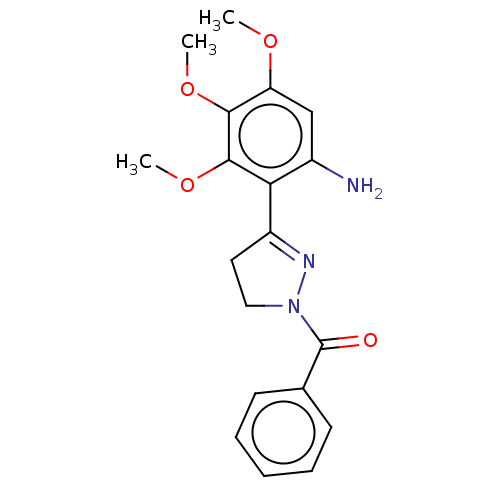
(CHEMBL2397625)Show SMILES COc1cc(N)c(C2=NN(CC2)C(=O)c2ccccc2)c(OC)c1OC |t:7| Show InChI InChI=1S/C19H21N3O4/c1-24-15-11-13(20)16(18(26-3)17(15)25-2)14-9-10-22(21-14)19(23)12-7-5-4-6-8-12/h4-8,11H,9-10,20H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of recombinant nNOS (unknown origin) assessed as conversion of L-[3H]-arginine to L-[3H]-citrulline after 30 mins |
Bioorg Med Chem 21: 4132-42 (2013)
Article DOI: 10.1016/j.bmc.2013.05.016
BindingDB Entry DOI: 10.7270/Q2CV4MPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50492296
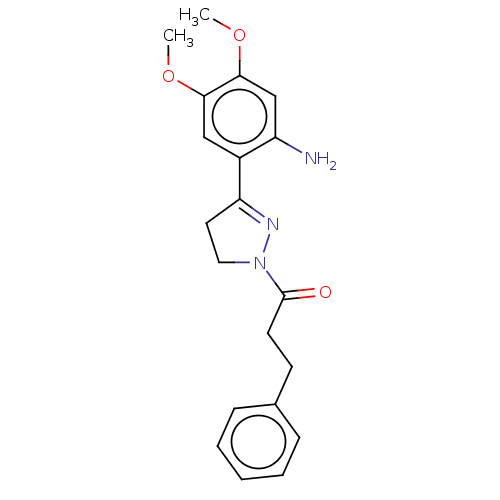
(CHEMBL2397623)Show SMILES COc1cc(N)c(cc1OC)C1=NN(CC1)C(=O)CCc1ccccc1 |t:12| Show InChI InChI=1S/C20H23N3O3/c1-25-18-12-15(16(21)13-19(18)26-2)17-10-11-23(22-17)20(24)9-8-14-6-4-3-5-7-14/h3-7,12-13H,8-11,21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of recombinant nNOS (unknown origin) assessed as conversion of L-[3H]-arginine to L-[3H]-citrulline after 30 mins |
Bioorg Med Chem 21: 4132-42 (2013)
Article DOI: 10.1016/j.bmc.2013.05.016
BindingDB Entry DOI: 10.7270/Q2CV4MPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50008990
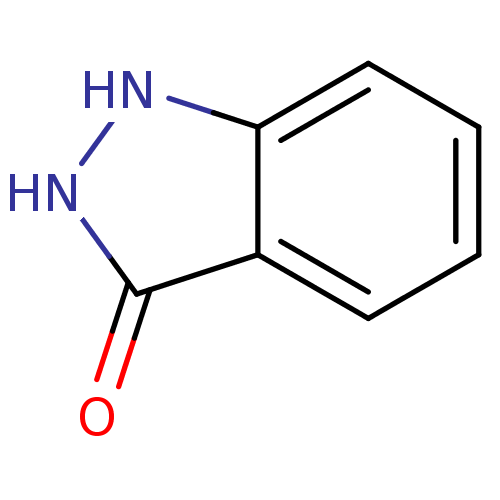
(1,2-Dihydro-indazol-3-one | 1H-indazol-3-ol | CHEM...)Show InChI InChI=1S/C7H6N2O/c10-7-5-3-1-2-4-6(5)8-9-7/h1-4H,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of nNOS in LPS-stimulated Wistar rat striata assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by liquid scintillati... |
Eur J Med Chem 46: 1439-47 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.027
BindingDB Entry DOI: 10.7270/Q2891659 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50304149
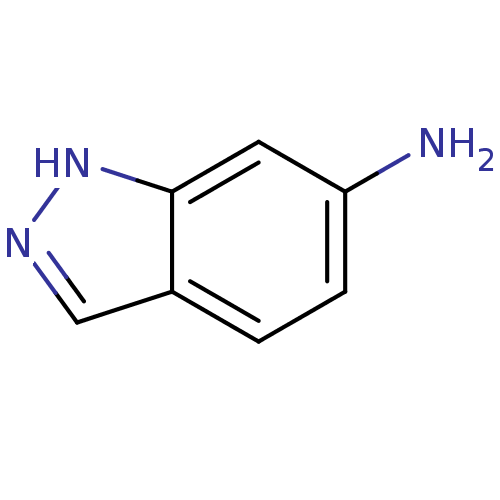
(1H-indazol-6-amine | CHEMBL594707 | cid_81423)Show InChI InChI=1S/C7H7N3/c8-6-2-1-5-4-9-10-7(5)3-6/h1-4H,8H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of recombinant NOS1 assessed as citrulline formation |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50008990

(1,2-Dihydro-indazol-3-one | 1H-indazol-3-ol | CHEM...)Show InChI InChI=1S/C7H6N2O/c10-7-5-3-1-2-4-6(5)8-9-7/h1-4H,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
UNED
Curated by ChEMBL
| Assay Description
Inhibition of recombinant NOS1 assessed as citrulline formation |
Bioorg Med Chem 17: 6180-7 (2009)
Article DOI: 10.1016/j.bmc.2009.07.067
BindingDB Entry DOI: 10.7270/Q2ZG6SBD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data