 Found 41538 hits with Last Name = 'che' and Initial = 'd'
Found 41538 hits with Last Name = 'che' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377655
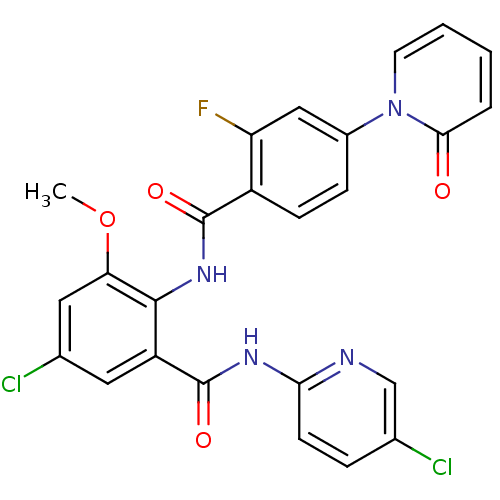
(CHEMBL260160)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1F)-n1ccccc1=O Show InChI InChI=1S/C25H17Cl2FN4O4/c1-36-20-11-15(27)10-18(25(35)30-21-8-5-14(26)13-29-21)23(20)31-24(34)17-7-6-16(12-19(17)28)32-9-3-2-4-22(32)33/h2-13H,1H3,(H,31,34)(H,29,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17135
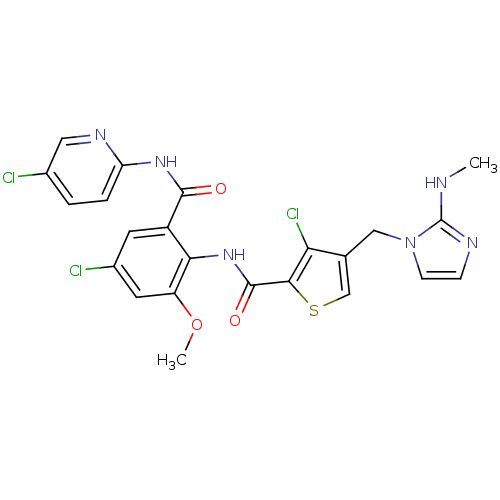
(3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...)Show SMILES CNc1nccn1Cc1csc(C(=O)Nc2c(OC)cc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c1Cl Show InChI InChI=1S/C23H19Cl3N6O3S/c1-27-23-28-5-6-32(23)10-12-11-36-20(18(12)26)22(34)31-19-15(7-14(25)8-16(19)35-2)21(33)30-17-4-3-13(24)9-29-17/h3-9,11H,10H2,1-2H3,(H,27,28)(H,31,34)(H,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377635
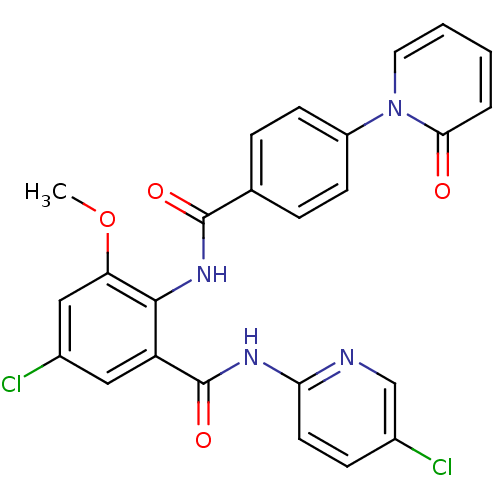
(CHEMBL402980)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H18Cl2N4O4/c1-35-20-13-17(27)12-19(25(34)29-21-10-7-16(26)14-28-21)23(20)30-24(33)15-5-8-18(9-6-15)31-11-3-2-4-22(31)32/h2-14H,1H3,(H,30,33)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471254
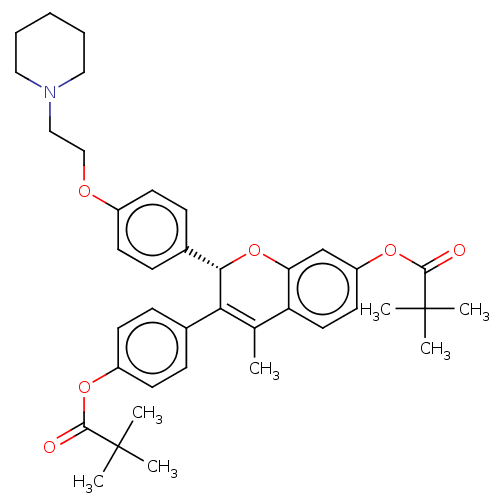
(CHEMBL308234)Show SMILES CC1=C([C@@H](Oc2cc(OC(=O)C(C)(C)C)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(OC(=O)C(C)(C)C)cc1 |r,t:1| Show InChI InChI=1S/C39H47NO6/c1-26-32-20-19-31(45-37(42)39(5,6)7)25-33(32)46-35(34(26)27-11-17-30(18-12-27)44-36(41)38(2,3)4)28-13-15-29(16-14-28)43-24-23-40-21-9-8-10-22-40/h11-20,25,35H,8-10,21-24H2,1-7H3/t35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1C
(Gallus gallus) | BDBM50470712
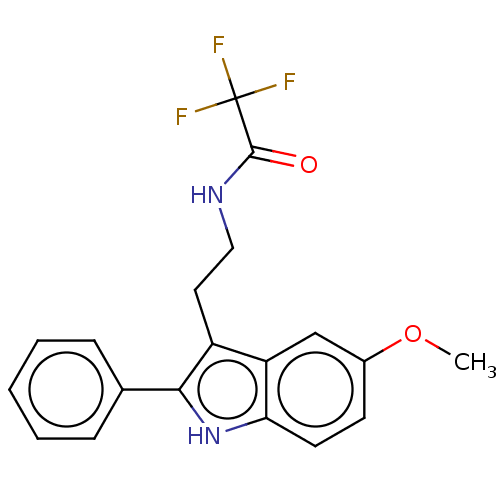
(CHEMBL417988)Show SMILES COc1ccc2[nH]c(c(CCNC(=O)C(F)(F)F)c2c1)-c1ccccc1 Show InChI InChI=1S/C19H17F3N2O2/c1-26-13-7-8-16-15(11-13)14(9-10-23-18(25)19(20,21)22)17(24-16)12-5-3-2-4-6-12/h2-8,11,24H,9-10H2,1H3,(H,23,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Binding affinity in chicken brain membranes by using [2-125I]melatonin in a competition radioligand binding assay |
J Med Chem 38: 1132-9 (1995)
Article DOI: 10.1021/jm00007a010
BindingDB Entry DOI: 10.7270/Q2HM5C6M |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373939
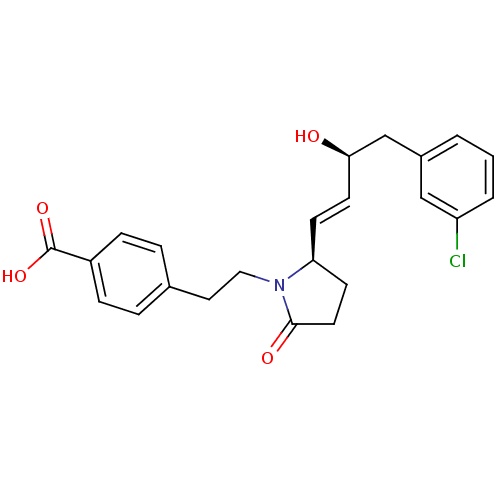
(CHEMBL258332)Show SMILES O[C@@H](Cc1cccc(Cl)c1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24ClNO4/c24-19-3-1-2-17(14-19)15-21(26)10-8-20-9-11-22(27)25(20)13-12-16-4-6-18(7-5-16)23(28)29/h1-8,10,14,20-21,26H,9,11-13,15H2,(H,28,29)/b10-8+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50243592
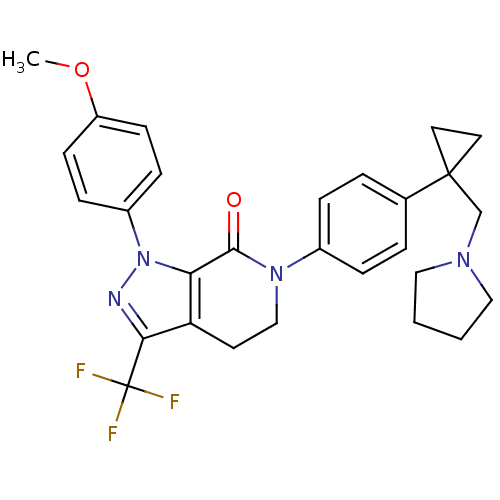
(1-(4-methoxyphenyl)-6-(4-(1-(pyrrolidin-1-ylmethyl...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)C1(CN2CCCC2)CC1)C(F)(F)F Show InChI InChI=1S/C28H29F3N4O2/c1-37-22-10-8-21(9-11-22)35-24-23(25(32-35)28(29,30)31)12-17-34(26(24)36)20-6-4-19(5-7-20)27(13-14-27)18-33-15-2-3-16-33/h4-11H,2-3,12-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4118-23 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.095
BindingDB Entry DOI: 10.7270/Q20Z7322 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471254

(CHEMBL308234)Show SMILES CC1=C([C@@H](Oc2cc(OC(=O)C(C)(C)C)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(OC(=O)C(C)(C)C)cc1 |r,t:1| Show InChI InChI=1S/C39H47NO6/c1-26-32-20-19-31(45-37(42)39(5,6)7)25-33(32)46-35(34(26)27-11-17-30(18-12-27)44-36(41)38(2,3)4)28-13-15-29(16-14-28)43-24-23-40-21-9-8-10-22-40/h11-20,25,35H,8-10,21-24H2,1-7H3/t35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Stimulation of alkaline phosphatase activity in human endometrial Ishikawa cells with 1 nM E2 estradiol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50243668
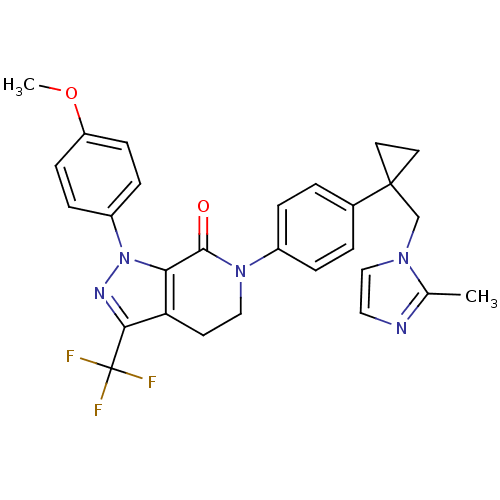
(1-(4-methoxyphenyl)-6-(4-(1-((2-methyl-1H-imidazol...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)C1(Cn2ccnc2C)CC1)C(F)(F)F Show InChI InChI=1S/C28H26F3N5O2/c1-18-32-14-16-34(18)17-27(12-13-27)19-3-5-20(6-4-19)35-15-11-23-24(26(35)37)36(33-25(23)28(29,30)31)21-7-9-22(38-2)10-8-21/h3-10,14,16H,11-13,15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4118-23 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.095
BindingDB Entry DOI: 10.7270/Q20Z7322 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471256
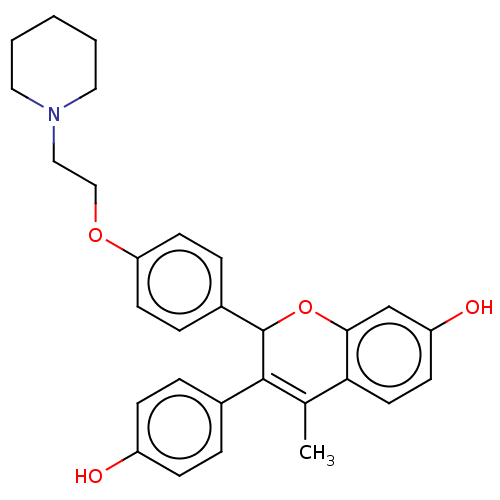
(CHEMBL291808)Show SMILES CC1=C(C(Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377637
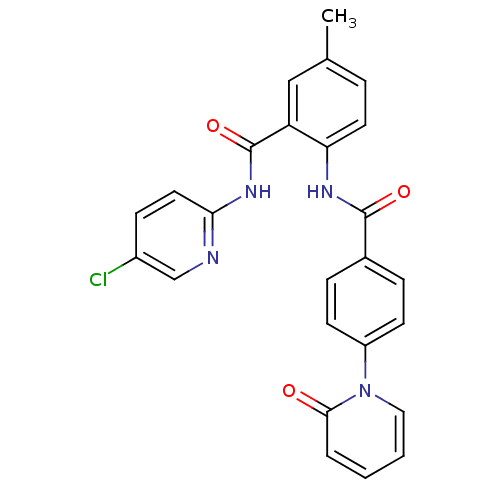
(CHEMBL257398)Show SMILES Cc1ccc(NC(=O)c2ccc(cc2)-n2ccccc2=O)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H19ClN4O3/c1-16-5-11-21(20(14-16)25(33)29-22-12-8-18(26)15-27-22)28-24(32)17-6-9-19(10-7-17)30-13-3-2-4-23(30)31/h2-15H,1H3,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12693
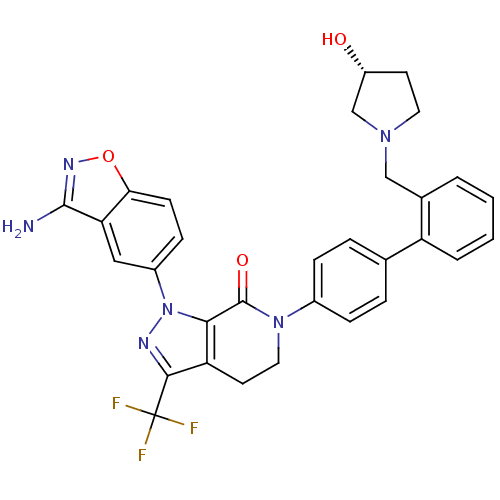
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H27F3N6O3/c32-31(33,34)28-24-12-14-39(30(42)27(24)40(36-28)21-9-10-26-25(15-21)29(35)37-43-26)20-7-5-18(6-8-20)23-4-2-1-3-19(23)16-38-13-11-22(41)17-38/h1-10,15,22,41H,11-14,16-17H2,(H2,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Replicase polyprotein 1ab
(BtCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| WIPO WO2021205298
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q232001P |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(BtCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| WIPO WO2021205298
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q232001P |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50167072
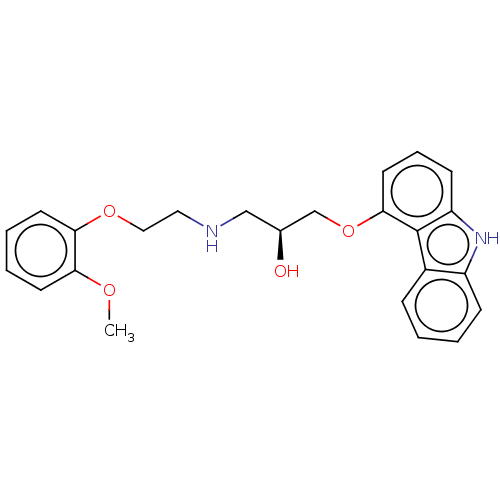
(CHEMBL3799125)Show SMILES COc1ccccc1OCCNC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C24H26N2O4/c1-28-21-10-4-5-11-22(21)29-14-13-25-15-17(27)16-30-23-12-6-9-20-24(23)18-7-2-3-8-19(18)26-20/h2-12,17,25-27H,13-16H2,1H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 receptor expressed in CHO cells |
Bioorg Med Chem 24: 2641-53 (2016)
Article DOI: 10.1016/j.bmc.2016.04.028
BindingDB Entry DOI: 10.7270/Q2ZC84S3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50243540
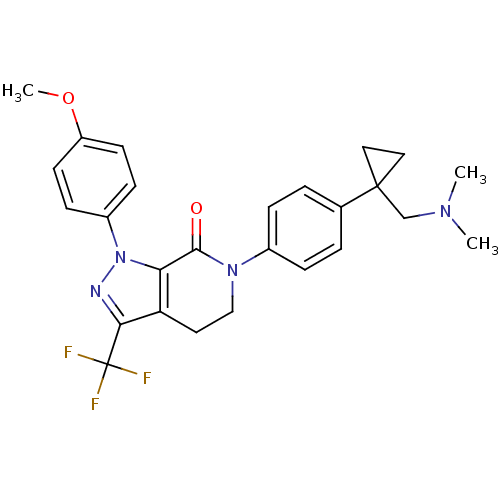
(6-(4-(1-((dimethylamino)methyl)cyclopropyl)phenyl)...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)C1(CN(C)C)CC1)C(F)(F)F Show InChI InChI=1S/C26H27F3N4O2/c1-31(2)16-25(13-14-25)17-4-6-18(7-5-17)32-15-12-21-22(24(32)34)33(30-23(21)26(27,28)29)19-8-10-20(35-3)11-9-19/h4-11H,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4118-23 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.095
BindingDB Entry DOI: 10.7270/Q20Z7322 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50145021
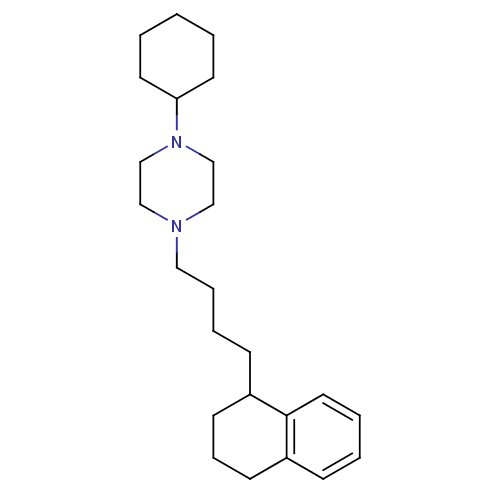
(1-Cyclohexyl-4-[4-(1,2,3,4-tetrahydro-naphthalen-1...)Show InChI InChI=1S/C24H38N2/c1-2-13-23(14-3-1)26-19-17-25(18-20-26)16-7-6-10-22-12-8-11-21-9-4-5-15-24(21)22/h4-5,9,15,22-23H,1-3,6-8,10-14,16-20H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Universit£t M£nster
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma1 receptor in guinea pig brain membranes |
Bioorg Med Chem 25: 4778-4799 (2017)
Article DOI: 10.1016/j.bmc.2017.07.027
BindingDB Entry DOI: 10.7270/Q26M3984 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50469380

(CHEMBL4276875)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C35H46N8O5/c1-21-16-25(44)17-22(2)26(21)20-27(36)32(46)41-28(14-9-15-40-35(38)39)33(47)43-30(19-24-12-7-4-8-13-24)34(48)42-29(31(37)45)18-23-10-5-3-6-11-23/h3-8,10-13,16-17,27-30,44H,9,14-15,18-20,36H2,1-2H3,(H2,37,45)(H,41,46)(H,42,48)(H,43,47)(H4,38,39,40)/t27-,28+,29-,30-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 60 mins by liquid scintillation counting |
J Med Chem 61: 9784-9789 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01282
BindingDB Entry DOI: 10.7270/Q2F76G8Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50243340
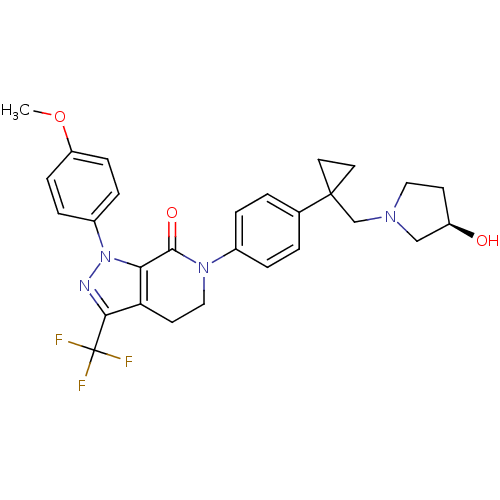
((R)-6-(4-(1-((3-hydroxypyrrolidin-1-yl)methyl)cycl...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)C1(CN2CC[C@@H](O)C2)CC1)C(F)(F)F |r| Show InChI InChI=1S/C28H29F3N4O3/c1-38-22-8-6-20(7-9-22)35-24-23(25(32-35)28(29,30)31)11-15-34(26(24)37)19-4-2-18(3-5-19)27(12-13-27)17-33-14-10-21(36)16-33/h2-9,21,36H,10-17H2,1H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4118-23 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.095
BindingDB Entry DOI: 10.7270/Q20Z7322 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50417287
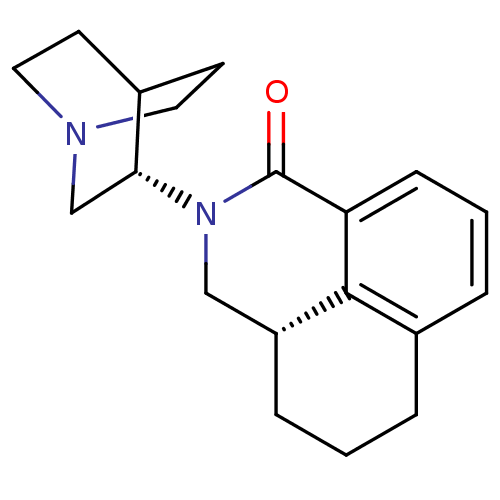
(Aloxi | Aurothioglucose | PALONOSETRON | PALONOSET...)Show SMILES O=C1N(C[C@H]2CCCc3cccc1c23)[C@@H]1CN2CCC1CC2 |wU:4.14,wD:14.16,(.24,-11.26,;.24,-12.8,;1.58,-13.56,;1.58,-15.1,;.24,-15.88,;.24,-17.42,;-1.09,-18.19,;-2.42,-17.42,;-2.42,-15.88,;-3.77,-15.1,;-3.77,-13.56,;-2.43,-12.78,;-1.09,-13.56,;-1.09,-15.1,;2.91,-12.8,;2.91,-11.26,;4.25,-10.49,;5.58,-11.26,;5.58,-12.8,;4.25,-13.56,;4.79,-12.41,;3.77,-11.79,)| Show InChI InChI=1S/C19H24N2O/c22-19-16-6-2-4-14-3-1-5-15(18(14)16)11-21(19)17-12-20-9-7-13(17)8-10-20/h2,4,6,13,15,17H,1,3,5,7-12H2/t15-,17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine |
J Med Chem 36: 2645-57 (1993)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2GM88T8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50110258
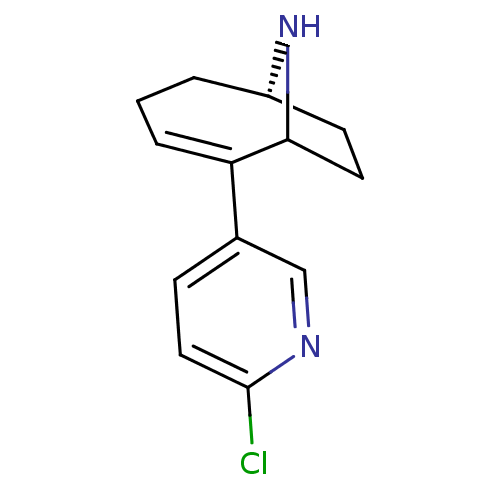
((6S)-2-(6-chloropyridin-3-yl)-9-azabicyclo[4.2.1]n...)Show SMILES Clc1ccc(cn1)C1=CCC[C@H]2CCC1N2 |t:8,THB:4:7:15:13.12| Show InChI InChI=1S/C13H15ClN2/c14-13-7-4-9(8-15-13)11-3-1-2-10-5-6-12(11)16-10/h3-4,7-8,10,12,16H,1-2,5-6H2/t10-,12?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from nAChR alpha4beta2 receptor |
Bioorg Med Chem Lett 22: 829-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.052
BindingDB Entry DOI: 10.7270/Q2GM87SD |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50243590
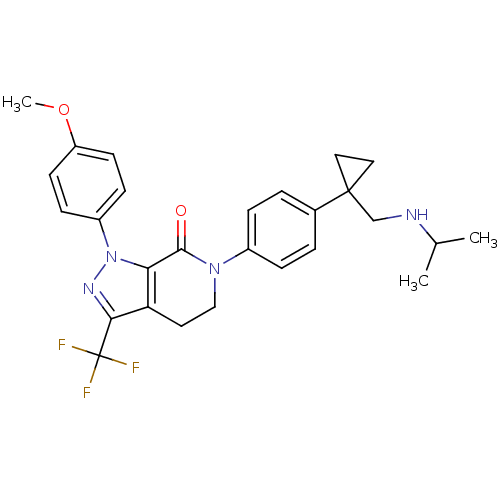
(6-(4-(1-((isopropylamino)methyl)cyclopropyl)phenyl...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)C1(CNC(C)C)CC1)C(F)(F)F Show InChI InChI=1S/C27H29F3N4O2/c1-17(2)31-16-26(13-14-26)18-4-6-19(7-5-18)33-15-12-22-23(25(33)35)34(32-24(22)27(28,29)30)20-8-10-21(36-3)11-9-20/h4-11,17,31H,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4118-23 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.095
BindingDB Entry DOI: 10.7270/Q20Z7322 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249295
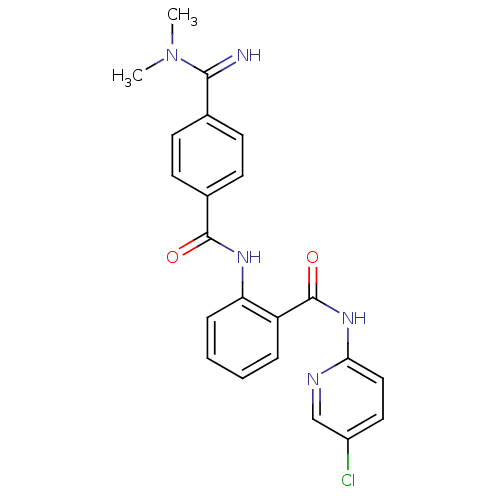
(CHEMBL471725 | N-(5-chloropyridin-2-yl)-2-(4-(N,N-...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccccc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H20ClN5O2/c1-28(2)20(24)14-7-9-15(10-8-14)21(29)26-18-6-4-3-5-17(18)22(30)27-19-12-11-16(23)13-25-19/h3-13,24H,1-2H3,(H,26,29)(H,25,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
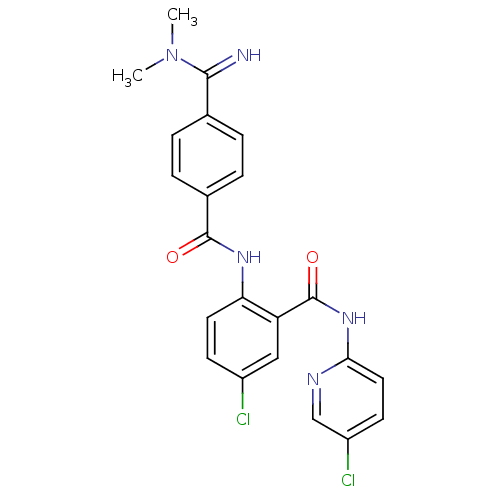
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1C
(Gallus gallus) | BDBM50470694
(CHEMBL14483)Show InChI InChI=1S/C20H22N2O2/c1-3-19(23)21-12-11-16-17-13-15(24-2)9-10-18(17)22-20(16)14-7-5-4-6-8-14/h4-10,13,22H,3,11-12H2,1-2H3,(H,21,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0466 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Binding affinity in chicken brain membranes by using [2-125I]melatonin in a competition radioligand binding assay |
J Med Chem 38: 1132-9 (1995)
Article DOI: 10.1021/jm00007a010
BindingDB Entry DOI: 10.7270/Q2HM5C6M |
More data for this
Ligand-Target Pair | |
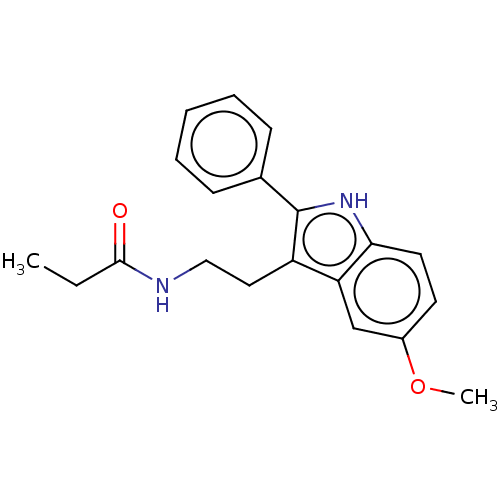
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377629
(CHEMBL260086)Show SMILES Oc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C24H20Cl2N4O4/c25-15-6-9-20(27-13-15)28-24(34)18-11-16(26)12-19(31)22(18)29-23(33)14-4-7-17(8-5-14)30-10-2-1-3-21(30)32/h4-9,11-13,31H,1-3,10H2,(H,29,33)(H,27,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
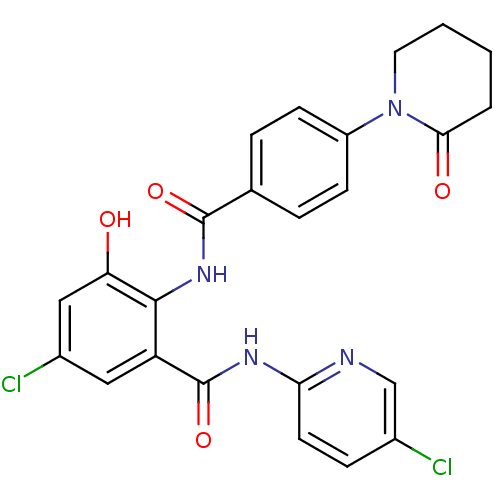
Coagulation factor X
(Homo sapiens (Human)) | BDBM50080514
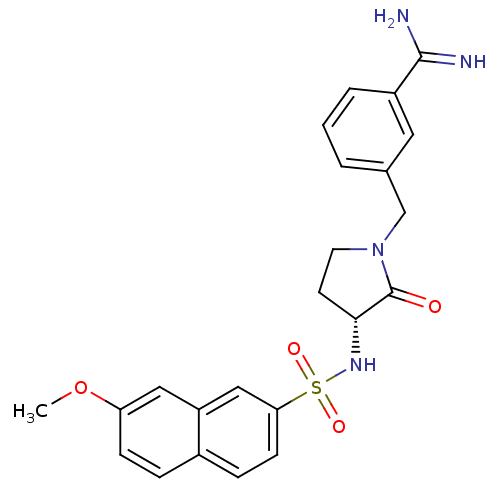
(3-[(R)-3-(7-Methoxy-naphthalene-2-sulfonylamino)-2...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C23H24N4O4S/c1-31-19-7-5-16-6-8-20(13-18(16)12-19)32(29,30)26-21-9-10-27(23(21)28)14-15-3-2-4-17(11-15)22(24)25/h2-8,11-13,21,26H,9-10,14H2,1H3,(H3,24,25)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of human Coagulation factor Xa |
J Med Chem 42: 3557-71 (1999)
Article DOI: 10.1021/jm990040h
BindingDB Entry DOI: 10.7270/Q2M04640 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373942
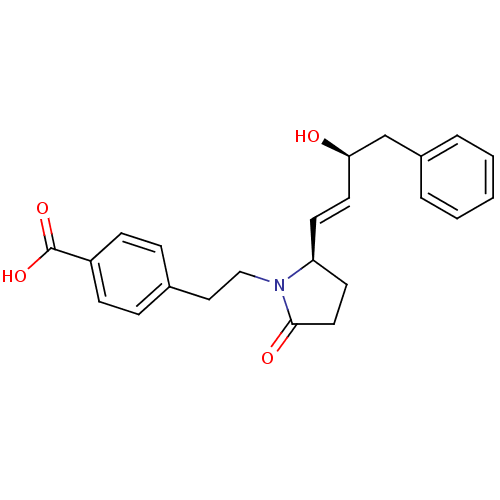
(CHEMBL272276)Show SMILES O[C@@H](Cc1ccccc1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO4/c25-21(16-18-4-2-1-3-5-18)12-10-20-11-13-22(26)24(20)15-14-17-6-8-19(9-7-17)23(27)28/h1-10,12,20-21,25H,11,13-16H2,(H,27,28)/b12-10+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50243630
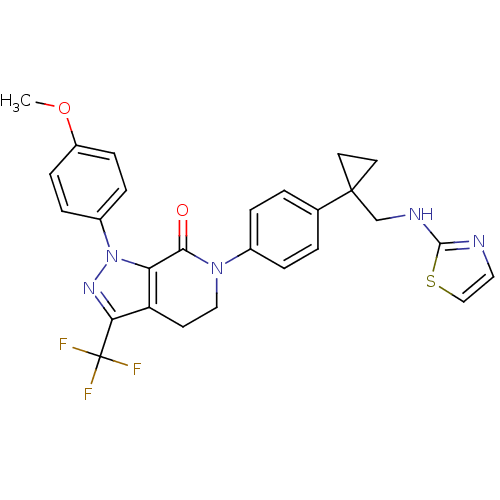
(1-(4-methoxyphenyl)-6-(4-(1-((thiazol-2-ylamino)me...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)C1(CNc2nccs2)CC1)C(F)(F)F Show InChI InChI=1S/C27H24F3N5O2S/c1-37-20-8-6-19(7-9-20)35-22-21(23(33-35)27(28,29)30)10-14-34(24(22)36)18-4-2-17(3-5-18)26(11-12-26)16-32-25-31-13-15-38-25/h2-9,13,15H,10-12,14,16H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4118-23 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.095
BindingDB Entry DOI: 10.7270/Q20Z7322 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50243669

(CHEMBL511486 | N-((1-(4-(1-(4-methoxyphenyl)-7-oxo...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)C1(CN(C)C(C)=O)CC1)C(F)(F)F Show InChI InChI=1S/C27H27F3N4O3/c1-17(35)32(2)16-26(13-14-26)18-4-6-19(7-5-18)33-15-12-22-23(25(33)36)34(31-24(22)27(28,29)30)20-8-10-21(37-3)11-9-20/h4-11H,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4118-23 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.095
BindingDB Entry DOI: 10.7270/Q20Z7322 |
More data for this
Ligand-Target Pair | |
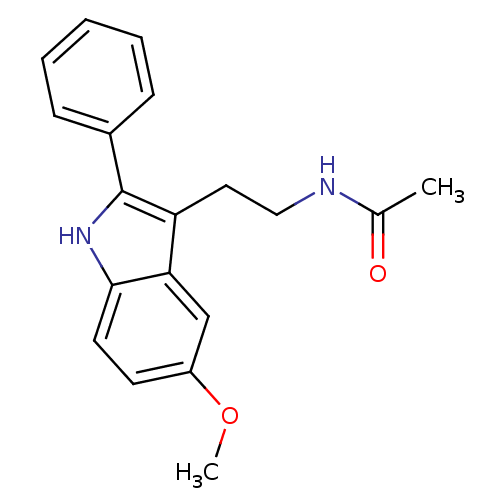
Melatonin receptor type 1C
(Gallus gallus) | BDBM50470711
(CHEMBL14403)Show InChI InChI=1S/C21H24N2O2/c1-3-7-20(24)22-13-12-17-18-14-16(25-2)10-11-19(18)23-21(17)15-8-5-4-6-9-15/h4-6,8-11,14,23H,3,7,12-13H2,1-2H3,(H,22,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0558 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Binding affinity in chicken brain membranes by using [2-125I]melatonin in a competition radioligand binding assay |
J Med Chem 38: 1132-9 (1995)
Article DOI: 10.1021/jm00007a010
BindingDB Entry DOI: 10.7270/Q2HM5C6M |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328717

(5-Chloro-N-(5-chloro-pyridin-2-yl)-3-methoxy-2-[4-...)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H22Cl2N4O4/c1-35-20-13-17(27)12-19(25(34)29-21-10-7-16(26)14-28-21)23(20)30-24(33)15-5-8-18(9-6-15)31-11-3-2-4-22(31)32/h5-10,12-14H,2-4,11H2,1H3,(H,30,33)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328717

(5-Chloro-N-(5-chloro-pyridin-2-yl)-3-methoxy-2-[4-...)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H22Cl2N4O4/c1-35-20-13-17(27)12-19(25(34)29-21-10-7-16(26)14-28-21)23(20)30-24(33)15-5-8-18(9-6-15)31-11-3-2-4-22(31)32/h5-10,12-14H,2-4,11H2,1H3,(H,30,33)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
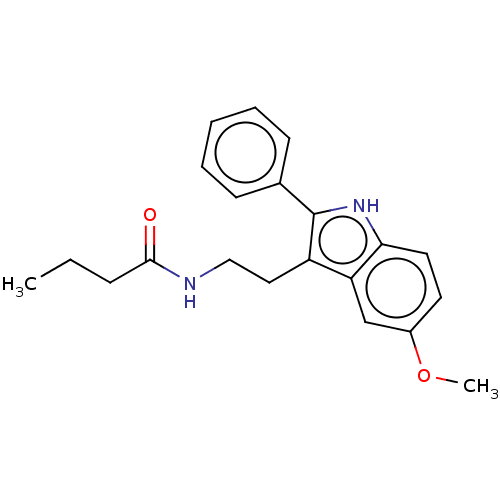
Melatonin receptor type 1C
(Gallus gallus) | BDBM50034110
(CHEMBL15060 | Melatonin,2-Phenyl | N-[2-(5-Methoxy...)Show InChI InChI=1S/C19H20N2O2/c1-13(22)20-11-10-16-17-12-15(23-2)8-9-18(17)21-19(16)14-6-4-3-5-7-14/h3-9,12,21H,10-11H2,1-2H3,(H,20,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0596 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Binding affinity in chicken brain membranes by using [2-125I]melatonin in a competition radioligand binding assay |
J Med Chem 38: 1132-9 (1995)
Article DOI: 10.1021/jm00007a010
BindingDB Entry DOI: 10.7270/Q2HM5C6M |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377638

(CHEMBL257400)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H16Cl2N4O3/c25-16-6-10-20(19(13-16)24(33)29-21-11-7-17(26)14-27-21)28-23(32)15-4-8-18(9-5-15)30-12-2-1-3-22(30)31/h1-14H,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50192770
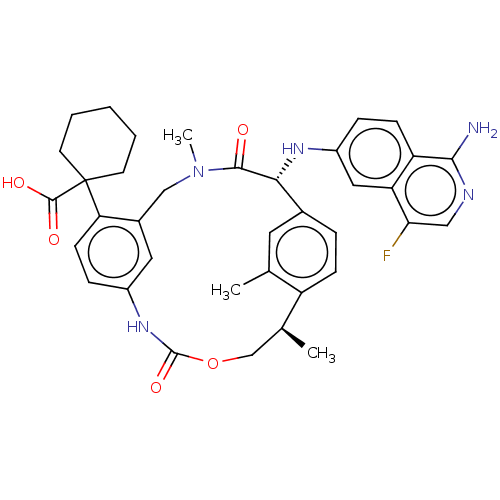
(CHEMBL3956096)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2)C1(CCCCC1)C(O)=O |r| Show InChI InChI=1S/C37H40FN5O5/c1-21-15-23-7-10-27(21)22(2)20-48-36(47)42-25-9-12-30(37(35(45)46)13-5-4-6-14-37)24(16-25)19-43(3)34(44)32(23)41-26-8-11-28-29(17-26)31(38)18-40-33(28)39/h7-12,15-18,22,32,41H,4-6,13-14,19-20H2,1-3H3,(H2,39,40)(H,42,47)(H,45,46)/t22-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TF-factor 7a using factor 10 as substrate at 37 degC |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50243628
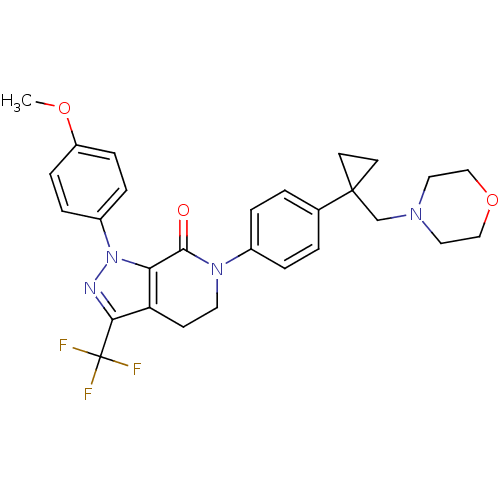
(1-(4-methoxyphenyl)-6-(4-(1-(morpholinomethyl)cycl...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)C1(CN2CCOCC2)CC1)C(F)(F)F Show InChI InChI=1S/C28H29F3N4O3/c1-37-22-8-6-21(7-9-22)35-24-23(25(32-35)28(29,30)31)10-13-34(26(24)36)20-4-2-19(3-5-20)27(11-12-27)18-33-14-16-38-17-15-33/h2-9H,10-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4118-23 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.095
BindingDB Entry DOI: 10.7270/Q20Z7322 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377628
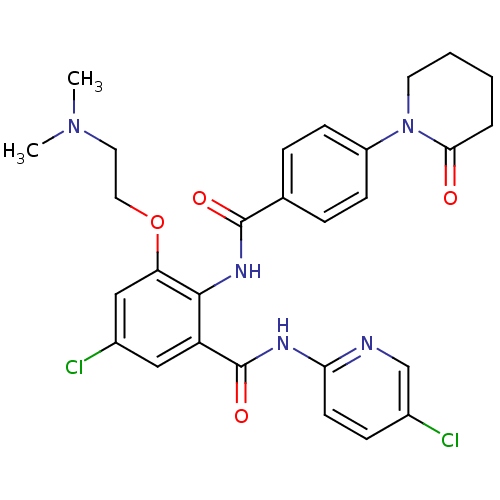
(CHEMBL261536)Show SMILES CN(C)CCOc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C28H29Cl2N5O4/c1-34(2)13-14-39-23-16-20(30)15-22(28(38)32-24-11-8-19(29)17-31-24)26(23)33-27(37)18-6-9-21(10-7-18)35-12-4-3-5-25(35)36/h6-11,15-17H,3-5,12-14H2,1-2H3,(H,33,37)(H,31,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50243670
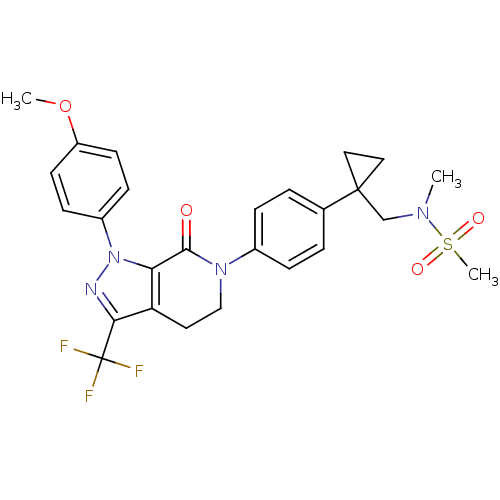
(CHEMBL471734 | N-((1-(4-(1-(4-methoxyphenyl)-7-oxo...)Show SMILES COc1ccc(cc1)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)C1(CN(C)S(C)(=O)=O)CC1)C(F)(F)F Show InChI InChI=1S/C26H27F3N4O4S/c1-31(38(3,35)36)16-25(13-14-25)17-4-6-18(7-5-17)32-15-12-21-22(24(32)34)33(30-23(21)26(27,28)29)19-8-10-20(37-2)11-9-19/h4-11H,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4118-23 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.095
BindingDB Entry DOI: 10.7270/Q20Z7322 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50365855
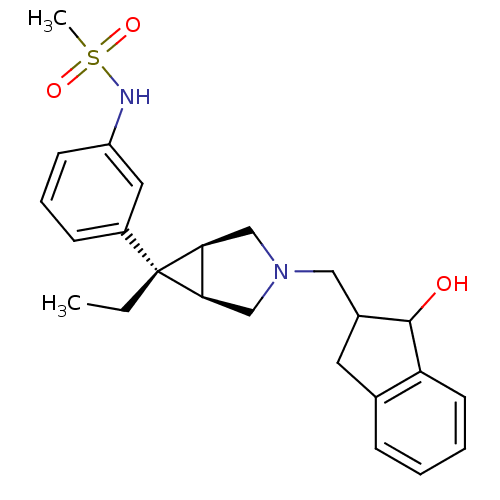
(CHEMBL1957843)Show SMILES CC[C@]1([C@H]2CN(CC3Cc4ccccc4C3O)C[C@@H]12)c1cccc(NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C24H30N2O3S/c1-3-24(18-8-6-9-19(12-18)25-30(2,28)29)21-14-26(15-22(21)24)13-17-11-16-7-4-5-10-20(16)23(17)27/h4-10,12,17,21-23,25,27H,3,11,13-15H2,1-2H3/t17?,21-,22+,23?,24+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor by GTP-gamma S binding assay |
Bioorg Med Chem Lett 22: 2200-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.099
BindingDB Entry DOI: 10.7270/Q2M90958 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
J Med Chem 53: 6243-74 (2010)
Article DOI: 10.1021/jm100146h
BindingDB Entry DOI: 10.7270/Q2CR5VBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data