

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM333146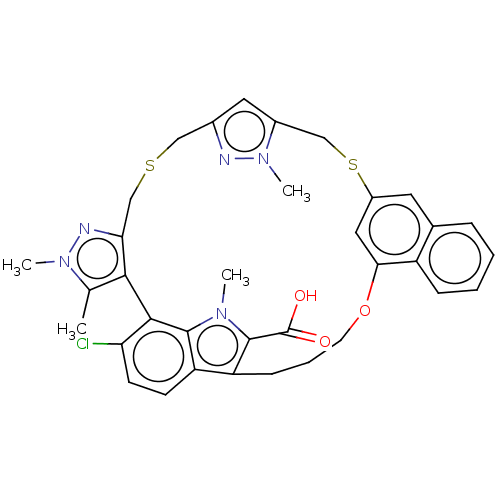 (Compound I | US10196404, Example 1 | US10196404, E...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was constructed such that GST tagged Mcl-1 protein, was incubated with a Europium-labeled anti-GST antibody and a HyLite Fluor 647-labeled ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MTR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM333146 (Compound I | US10196404, Example 1 | US10196404, E...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The assay was constructed such that GST tagged Mcl-1 protein, was incubated with a Europium-labeled anti-GST antibody and a HyLite Fluor 647-labeled ... | US Patent US10196404 (2019) BindingDB Entry DOI: 10.7270/Q2P2717Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM333146 (Compound I | US10196404, Example 1 | US10196404, E...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was constructed such that GST tagged Mcl-1 protein, was incubated with a Europium-labeled anti-GST antibody and a HyLite Fluor 647-labeled ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MTR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM333146 (Compound I | US10196404, Example 1 | US10196404, E...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The assay was constructed such that GST tagged Mcl-1 protein, was incubated with a Europium-labeled anti-GST antibody and a HyLite Fluor 647-labeled ... | US Patent US10196404 (2019) BindingDB Entry DOI: 10.7270/Q2P2717Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50249522 (2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(me...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385637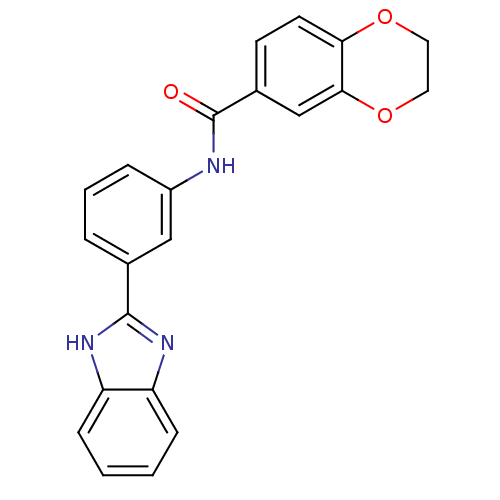 (CHEMBL2043430) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385635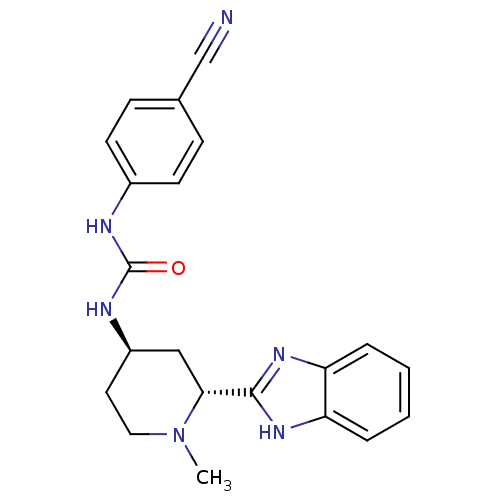 (CHEMBL2043437) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385642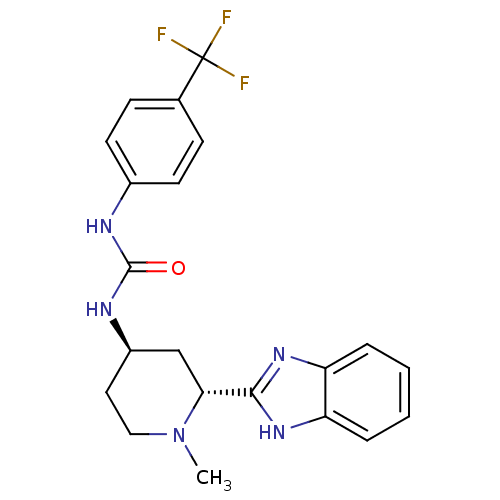 (CHEMBL2043435) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216194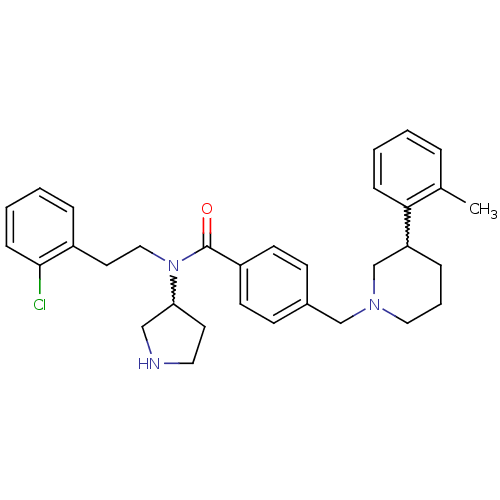 (CHEMBL391935 | N-(2-chlorophenethyl)-N-(pyrrolidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385643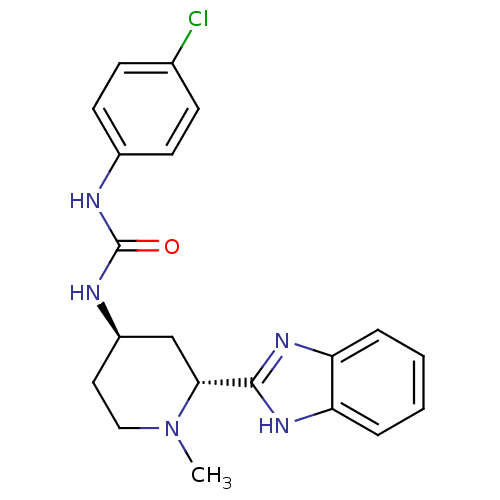 (CHEMBL2043436) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052137 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216201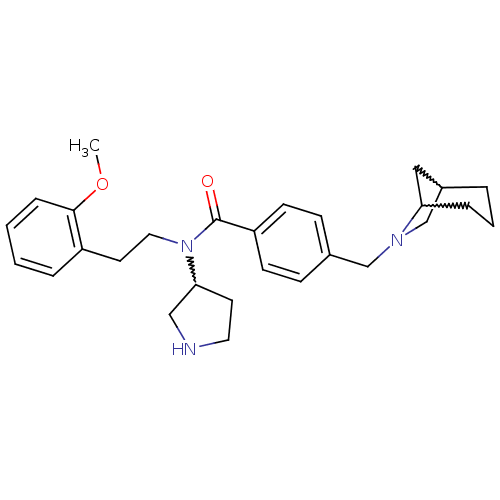 (CHEMBL233987 | N-(2-methoxyphenethyl)-4-(6-aza-bic...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50010274 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-(2-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052124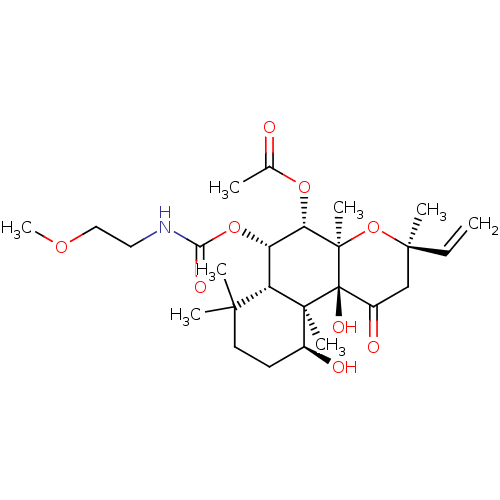 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385641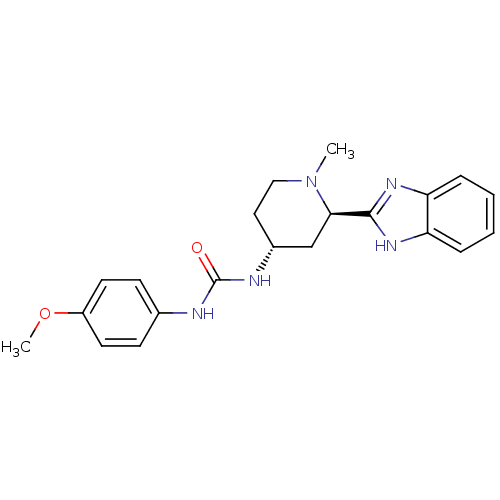 (CHEMBL2043434) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052123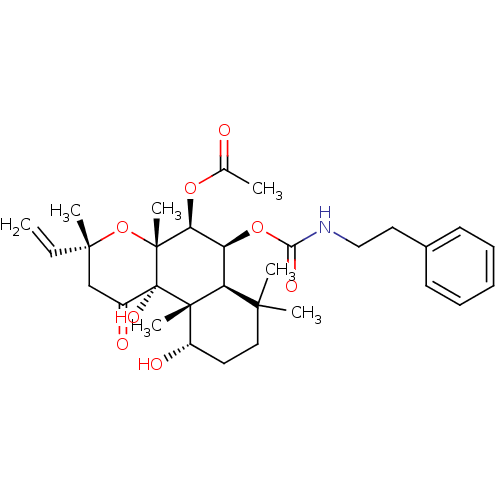 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216180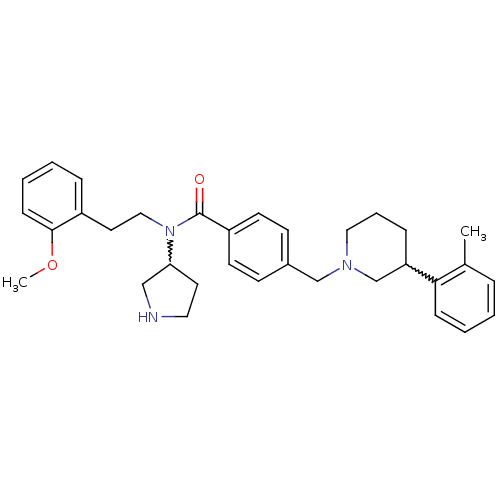 (CHEMBL233799 | N-(2-methoxyphenethyl)-N-(pyrrolidi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052141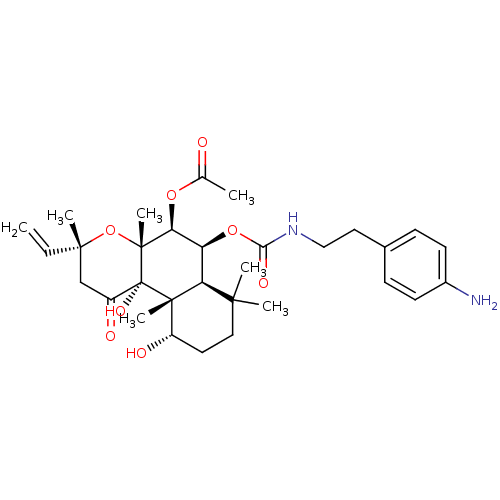 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-[2-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50010261 ((3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-6,10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052142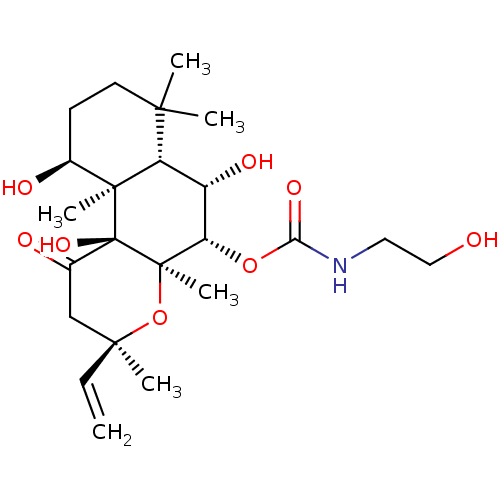 ((2-Hydroxy-ethyl)-carbamic acid (3R,4aR,5S,6S,6aS,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385638 (CHEMBL2043432) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052136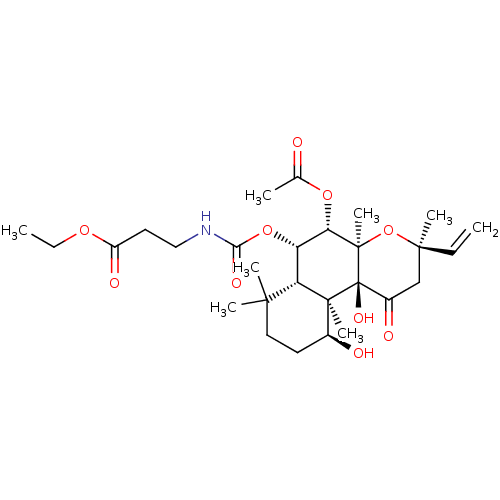 (3-((3R,4aR,5S,6S,6aS,10S,10aR,10bS)-5-Acetoxy-10,1...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385640 (CHEMBL2043433) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052143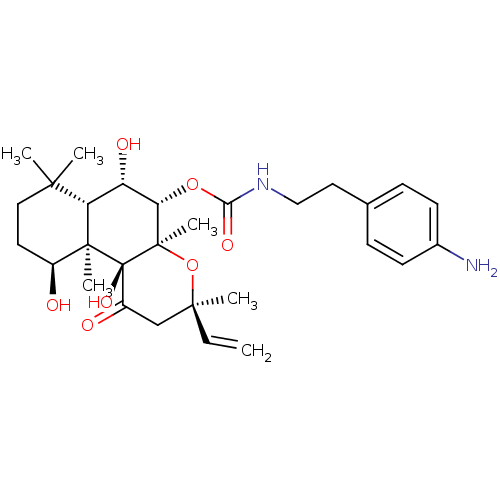 (CHEMBL93242 | [2-(4-Amino-phenyl)-ethyl]-carbamic ...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052129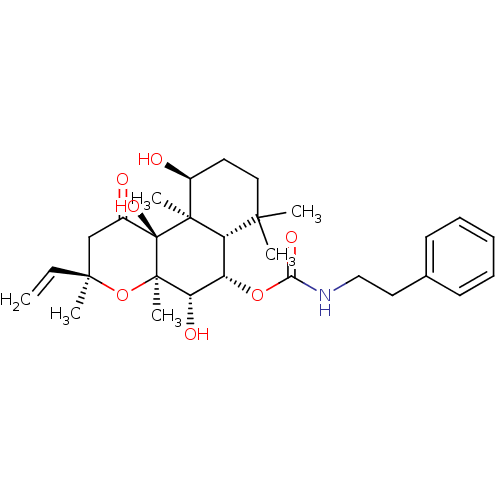 (CHEMBL93259 | Phenethyl-carbamic acid (3R,4aR,5S,6...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM333146 (Compound I | US10196404, Example 1 | US10196404, E...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was constructed such that GST tagged Mcl-1 protein, was incubated with a Europium-labeled anti-GST antibody and a HyLite Fluor 647-labeled ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2959MTR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM333146 (Compound I | US10196404, Example 1 | US10196404, E...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The assay was constructed such that GST tagged Mcl-1 protein, was incubated with a Europium-labeled anti-GST antibody and a HyLite Fluor 647-labeled ... | US Patent US10196404 (2019) BindingDB Entry DOI: 10.7270/Q2P2717Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385644 (CHEMBL2043438) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052132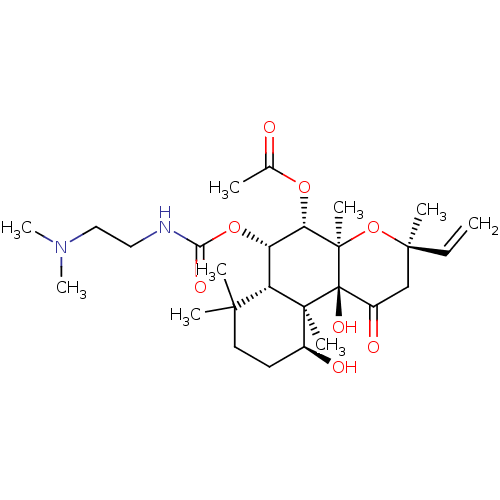 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-(2-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052149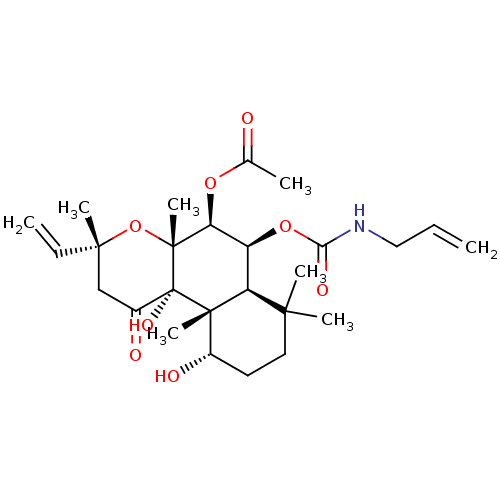 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-all...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052127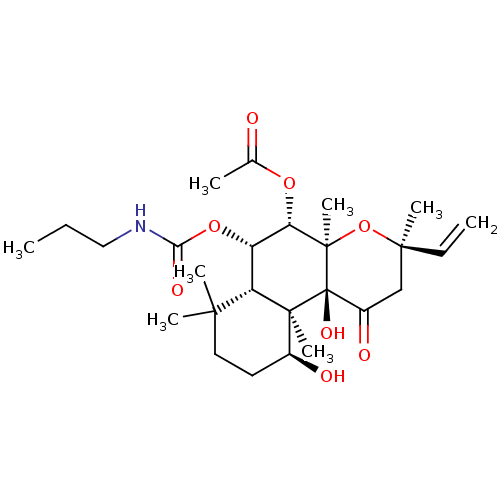 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052140 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385639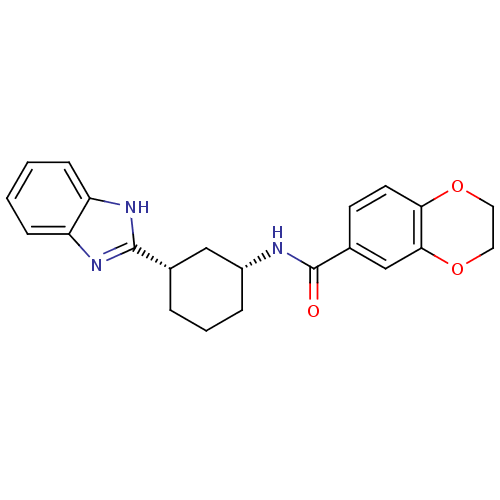 (CHEMBL2043431) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052131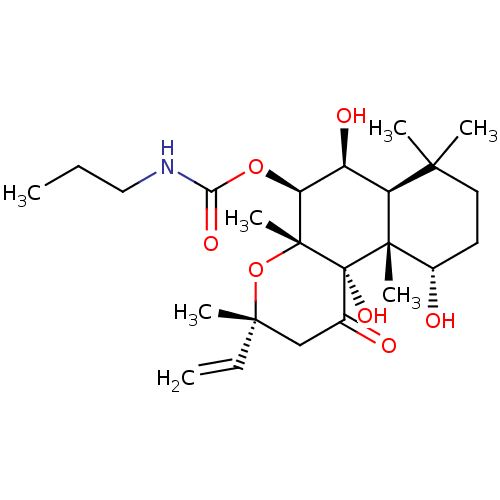 (CHEMBL93521 | Propyl-carbamic acid (3R,4aR,5S,6S,6...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50010269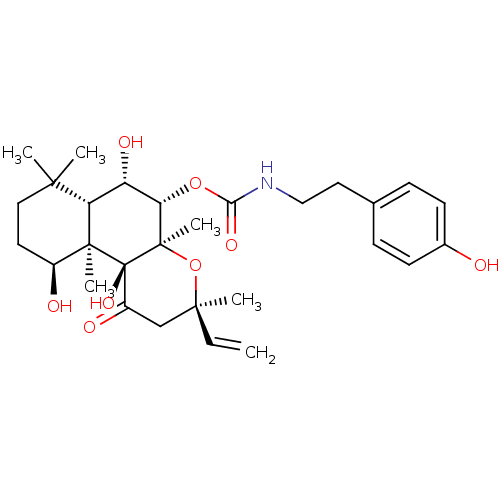 (CHEMBL327672 | [2-(4-Hydroxy-phenyl)-ethyl]-carbam...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216199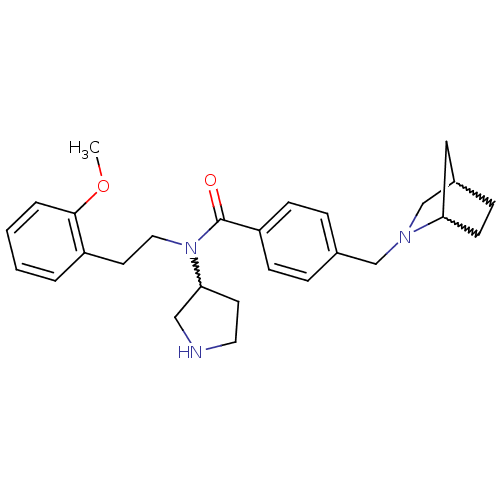 (4-(2-aza-bicyclo[2.2.1]heptan-2-ylmethyl)-N-(2-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216188 (CHEMBL233422 | N-(2-methoxyphenethyl)-4-(((1S,2S)-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50385636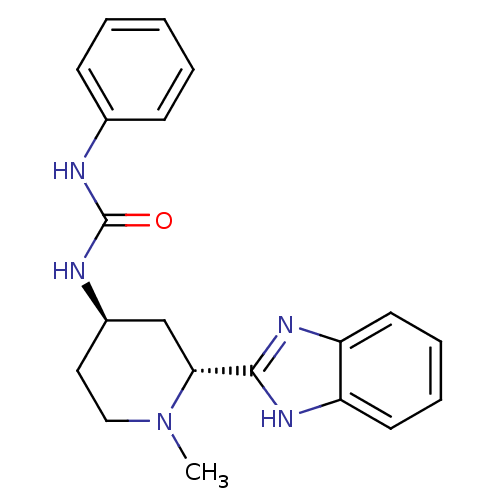 (CHEMBL2043429) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr... | ACS Med Chem Lett 3: 106-111 (2012) Article DOI: 10.1021/ml2002423 BindingDB Entry DOI: 10.7270/Q2W096Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052116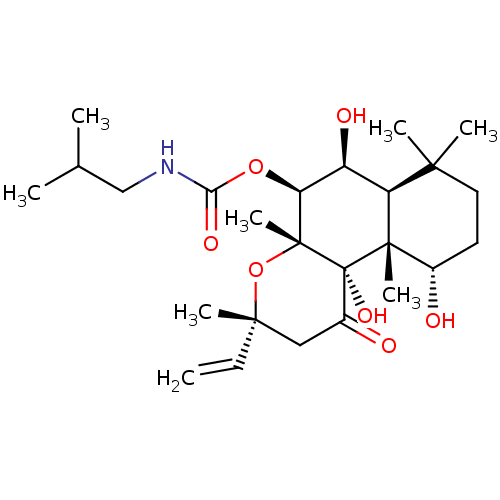 (CHEMBL93260 | Isobutyl-carbamic acid (3R,4aR,5S,6S...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052146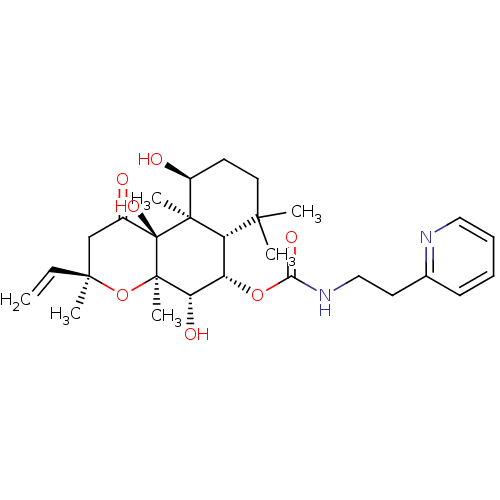 ((2-Pyridin-2-yl-ethyl)-carbamic acid (3R,4aR,5S,6S...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052133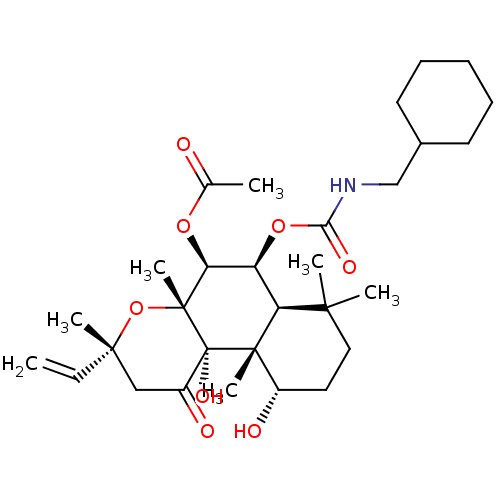 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-cyc...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052148 ((2-Piperidin-1-yl-ethyl)-carbamic acid (3R,4aR,5S,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50010268 ((2-Amino-ethyl)-carbamic acid (3R,4aR,5S,6S,6aS,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane-bound transcription factor site-1 protease (Homo sapiens (Human)) | BDBM50216193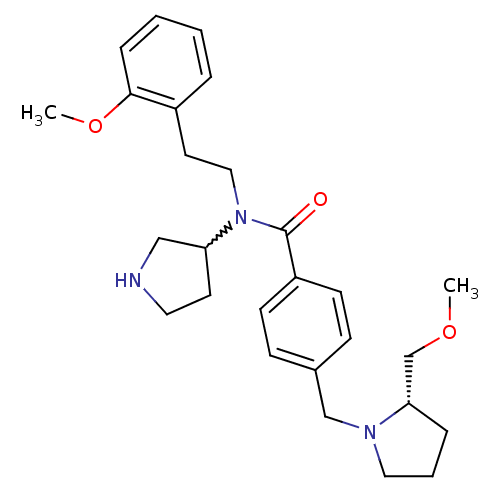 (CHEMBL233609 | N-(2-methoxyphenethyl)-4-(((S)-2-(m...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Inhibition of human S1P expressed in CHOK1 cells | Bioorg Med Chem Lett 17: 4411-4 (2007) Article DOI: 10.1016/j.bmcl.2007.06.031 BindingDB Entry DOI: 10.7270/Q26M36JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052150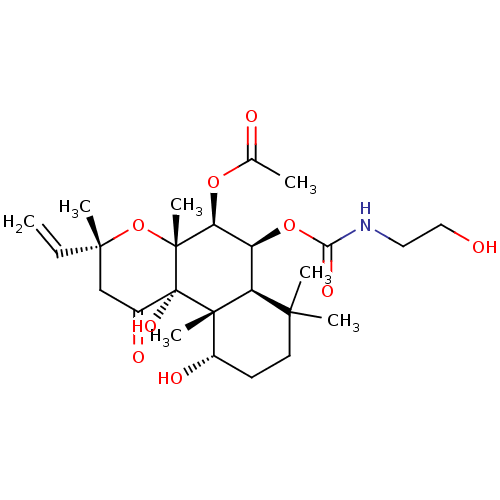 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052130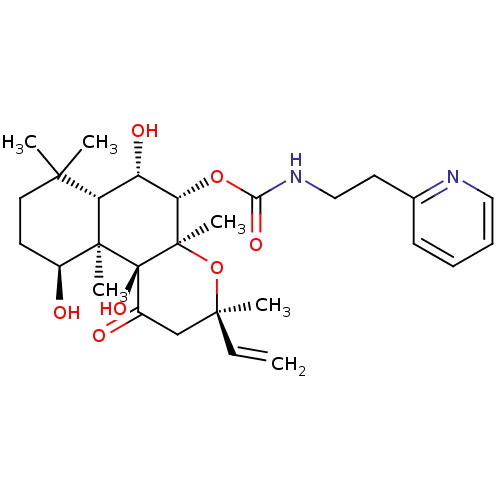 ((2-Pyridin-2-yl-ethyl)-carbamic acid (3R,4aR,5S,6S...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052133 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-cyc...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >150 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052111 ((2-Methoxy-ethyl)-carbamic acid (3R,4aR,5S,6S,6aS,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >150 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50010267 (CHEMBL92719 | [2-(4-Hydroxy-phenyl)-ethyl]-carbami...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >150 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052121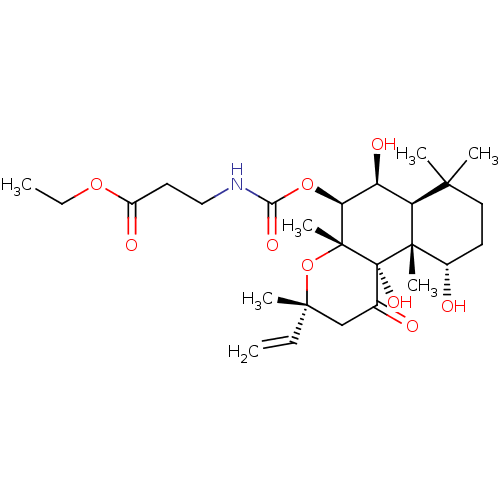 (3-((3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6,10,10b-Trihy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >150 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 240 total ) | Next | Last >> |