

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50304090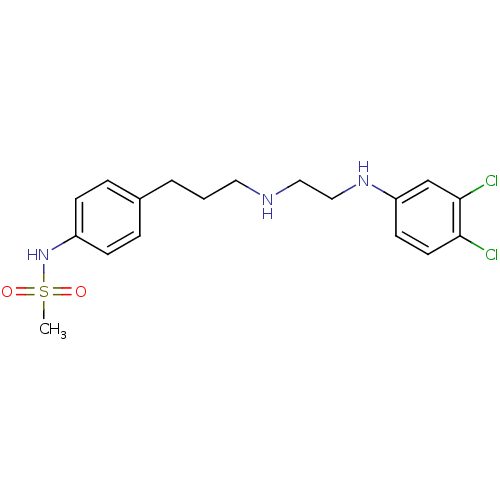 (CHEMBL594615 | N-(4-(3-(2-(3,4-Dichlorophenylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50304085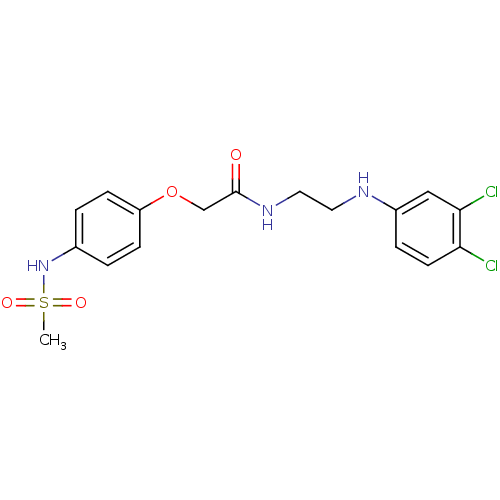 (CHEMBL607819 | N-(2-(3,4-Dichlorophenylamino)ethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membrane | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50304088 (CHEMBL596046 | N-(4-(2-(2-(3,4-Dichlorophenylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50304087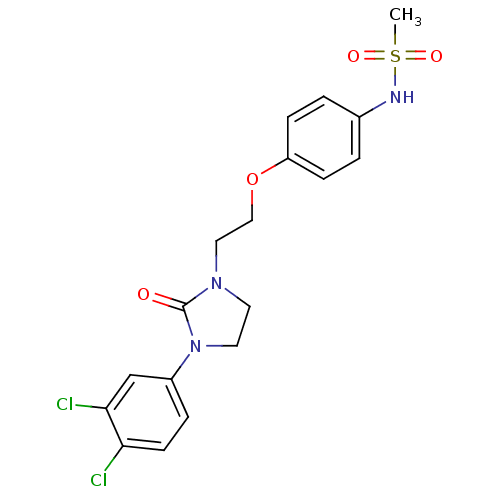 (CHEMBL596036 | N-(4-(2-(3-(3,4-Dichlorophenyl)-2-o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membrane | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168618 (US9079852, Table F, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 553 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50304086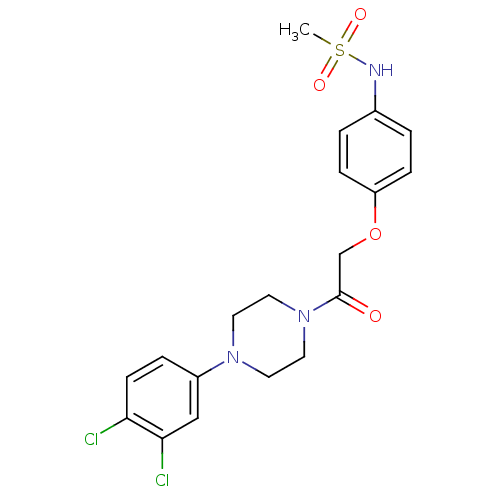 (CHEMBL594417 | N-(4-(2-(4-(3,4-Dichlorophenyl)pipe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 817 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]ifenprodil form NR2B receptor in Wistar rat cerebral cortex membrane | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168620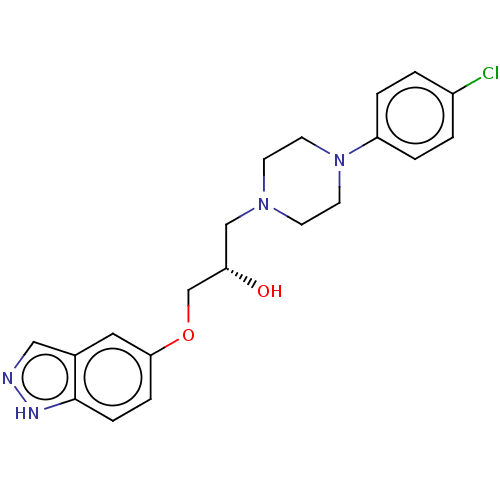 (US9079852, Table F, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168617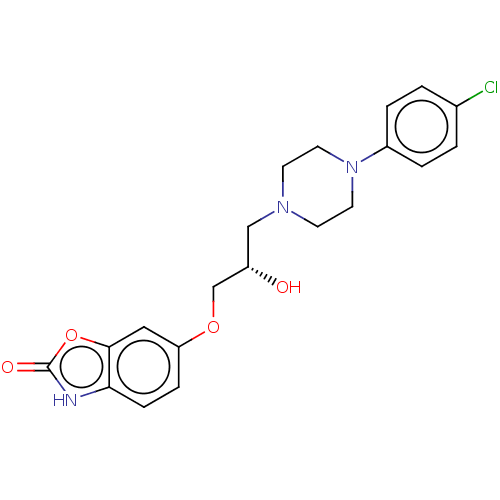 (US9079852, Table F, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50304091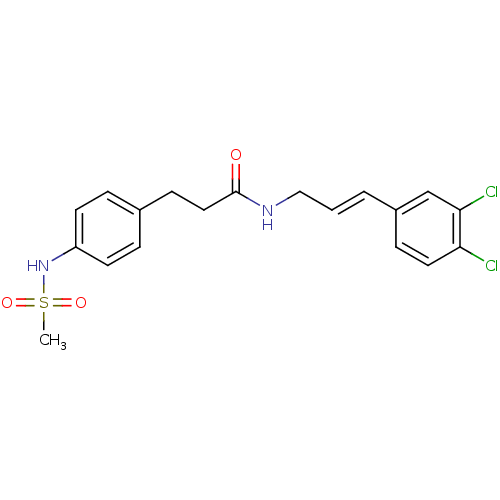 (CHEMBL609857 | N-(3,4-Dichlorocinnamyl)-3-(4-(meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50304089 (CHEMBL595318 | N-(2-(3,4-Dichlorophenylamino)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]astemizole from human recombinant ERG expressed in HEK293 cells | Bioorg Med Chem 17: 6463-80 (2009) Article DOI: 10.1016/j.bmc.2009.05.085 BindingDB Entry DOI: 10.7270/Q2M61KB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168626 (US9079852, Table F, Compound 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168622 (US9079852, Table F, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168621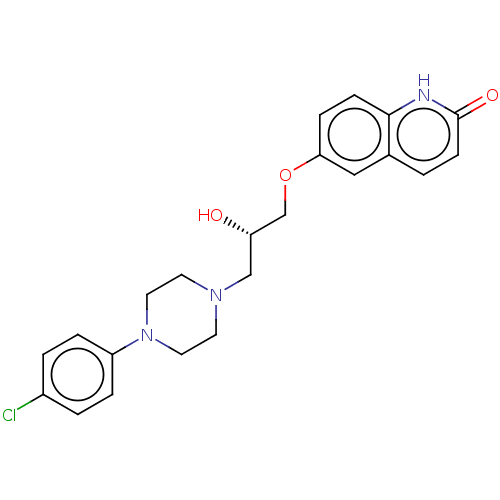 (US9079852, Table F, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168625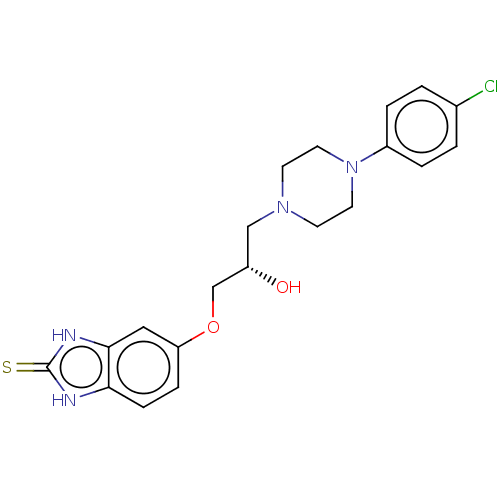 (US9079852, Table F, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168624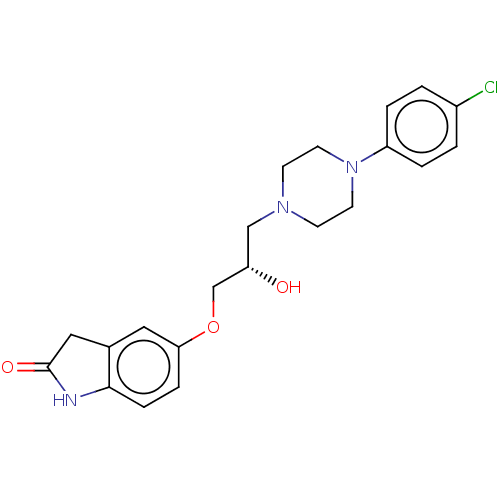 (US9079852, Table F, Compound 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50440057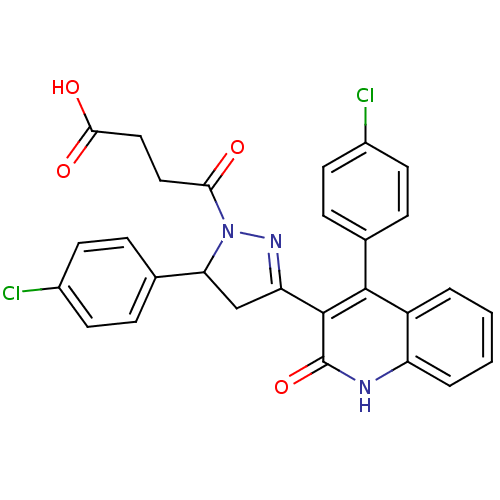 (CHEMBL2426097) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor (unknown origin) | J Med Chem 56: 6434-56 (2013) Article DOI: 10.1021/jm400652r BindingDB Entry DOI: 10.7270/Q26H4JTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168619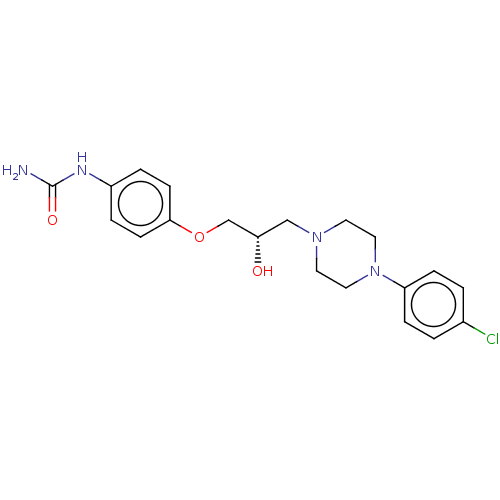 (US9079852, Table F, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168616 (US9079852, Table F, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50538441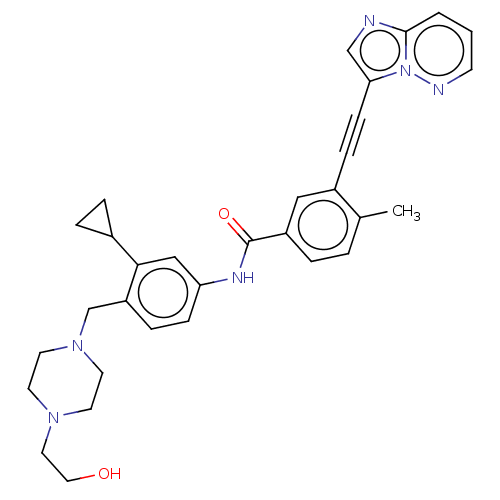 (CHEMBL4632471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human ABL1 assessed as residual activity using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50322535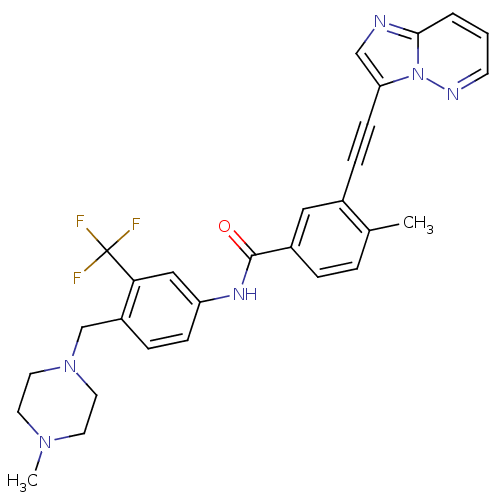 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human ABL1 assessed as residual activity using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50538440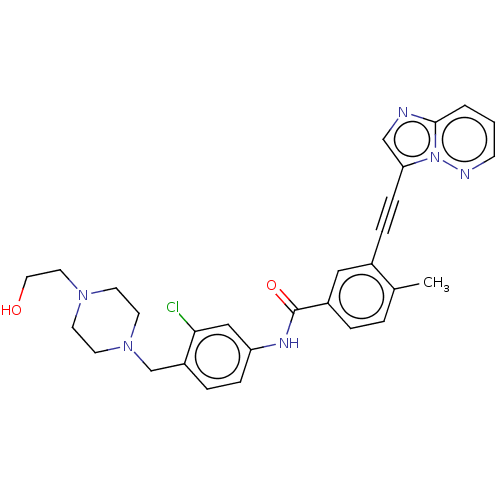 (CHEMBL4642144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human ABL1 assessed as residual activity using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50225416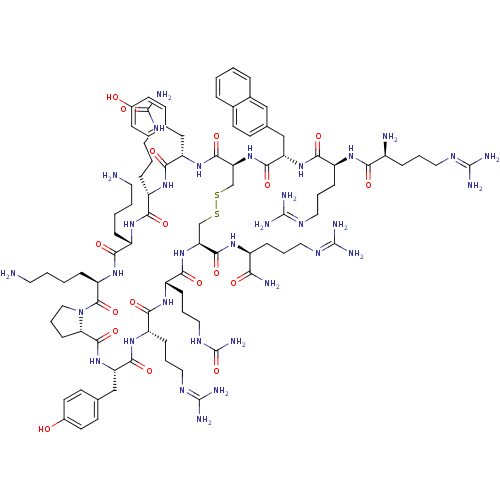 (CHEMBL393882 | TN-14003) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of CXCR4 in MDA-MB-231 cells | J Med Chem 50: 5655-64 (2007) Article DOI: 10.1021/jm070679i BindingDB Entry DOI: 10.7270/Q2X066RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50538439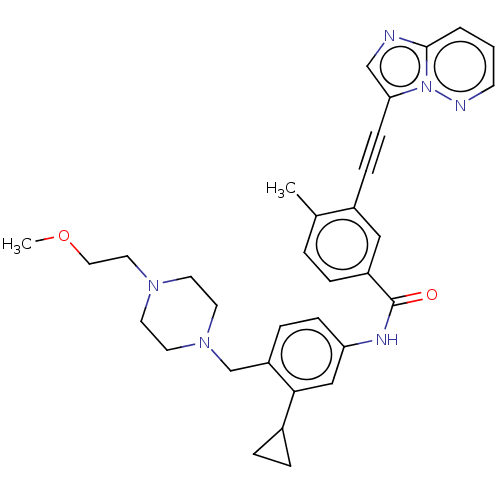 (CHEMBL4637502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human ABL1 assessed as residual activity using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50347490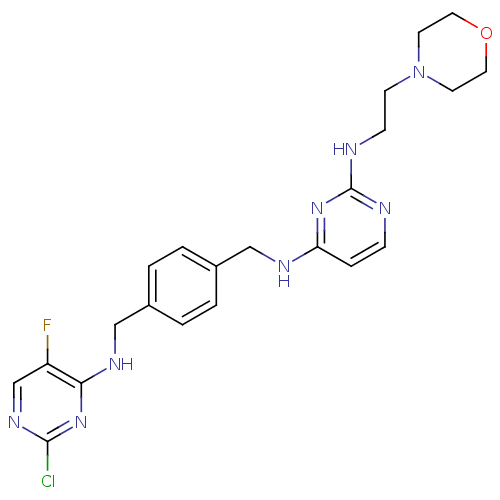 (CHEMBL1802333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo... | J Med Chem 53: 8556-68 (2010) Article DOI: 10.1021/jm100786g BindingDB Entry DOI: 10.7270/Q2PK0GH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443545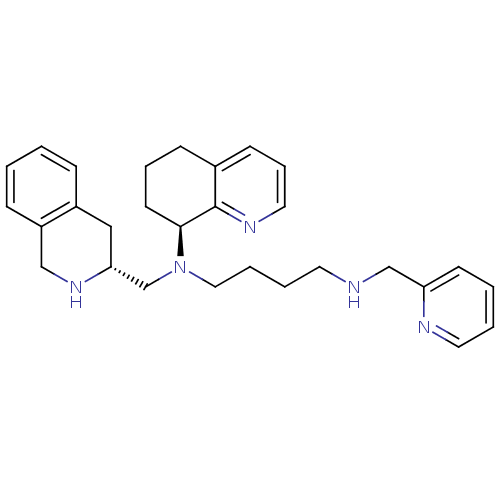 (CHEMBL3091683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50538442 (CHEMBL4638981) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of ABL1 (unknown origin) assessed as residual activity incubated for 5 mins in presence of [gamma-33ATP] by scintillation counting based r... | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50225415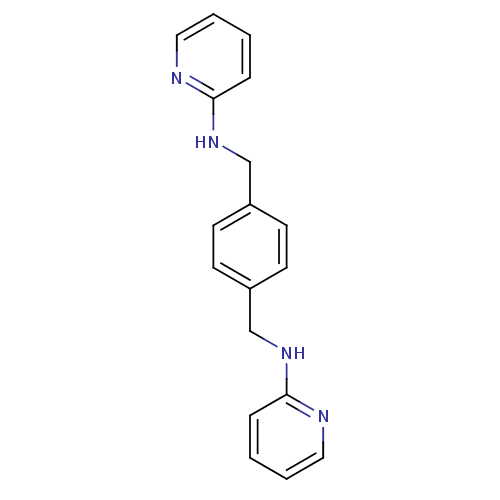 (CHEMBL237830 | N,N'-di-2-pyridinyl-1,4-benzenedime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo... | J Med Chem 53: 8556-68 (2010) Article DOI: 10.1021/jm100786g BindingDB Entry DOI: 10.7270/Q2PK0GH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50538442 (CHEMBL4638981) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha (unknown origin) assessed as residual activity incubated for 5 mins in presence of [gamma-33ATP] by scintillation counting b... | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125954 (CHEMBL3627858) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125950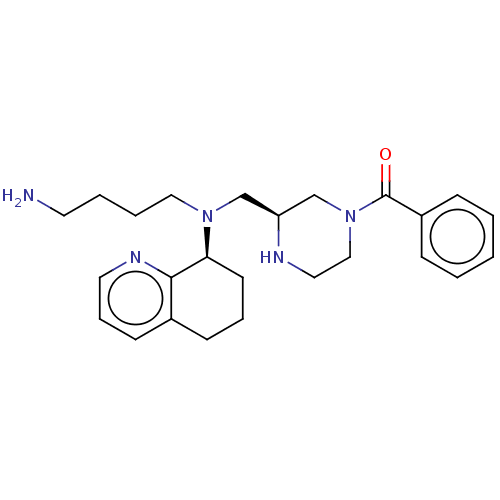 (CHEMBL3627793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125958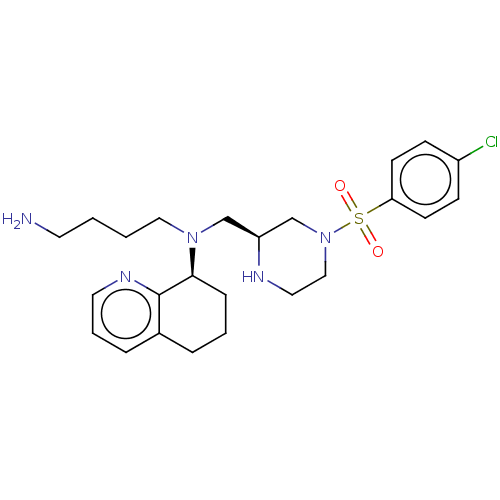 (CHEMBL3627862) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50538438 (CHEMBL4640297) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha (unknown origin) assessed as residual activity incubated for 5 mins in presence of [gamma-33ATP] by scintillation counting b... | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50449948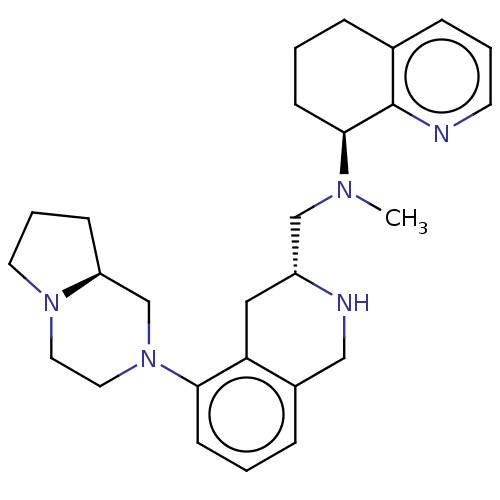 (CHEMBL4173977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins followed b... | J Med Chem 61: 7168-7188 (2018) Article DOI: 10.1021/acs.jmedchem.8b00450 BindingDB Entry DOI: 10.7270/Q2GH9MJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541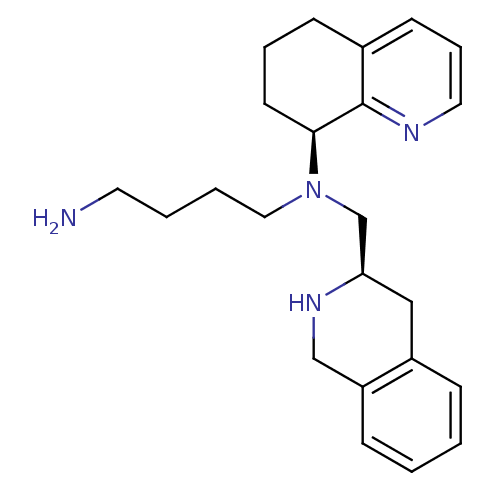 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50347388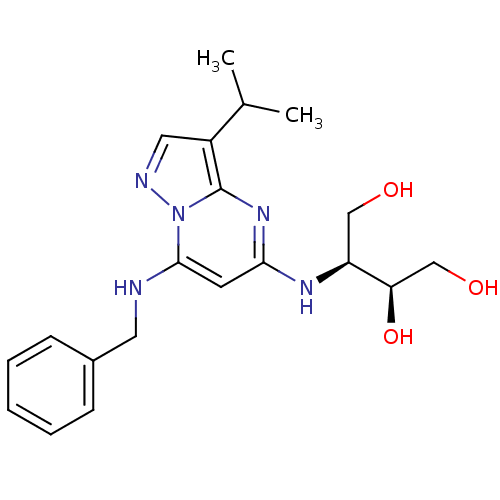 (CHEMBL1234833) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclinE assessed as amount of ATP released by luciferase activity based PKLight assay | J Med Chem 53: 8508-22 (2010) Article DOI: 10.1021/jm100732t BindingDB Entry DOI: 10.7270/Q2B858GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443543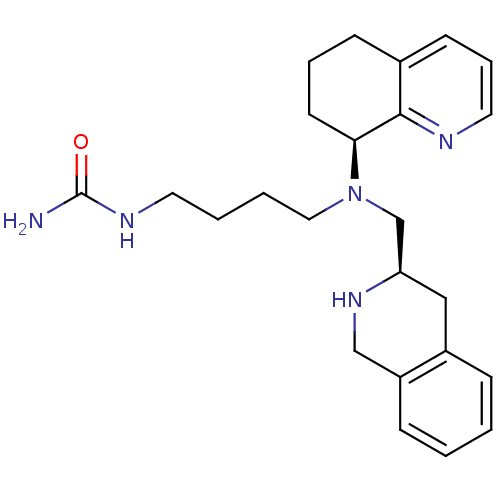 (CHEMBL3091685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50538438 (CHEMBL4640297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of ABL1 (unknown origin) assessed as residual activity incubated for 5 mins in presence of [gamma-33ATP] by scintillation counting based r... | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50449934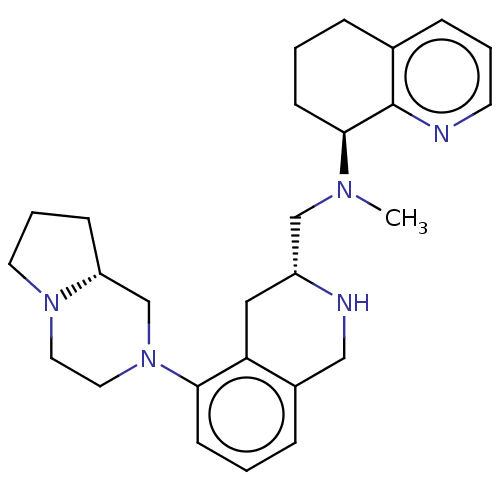 (CHEMBL4162609) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins followed b... | J Med Chem 61: 7168-7188 (2018) Article DOI: 10.1021/acs.jmedchem.8b00450 BindingDB Entry DOI: 10.7270/Q2GH9MJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270038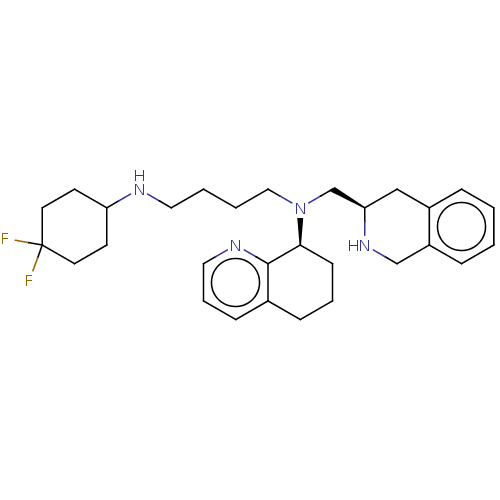 (CHEMBL4062223) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443544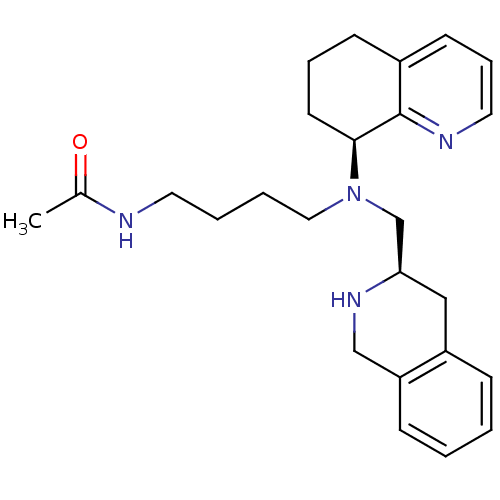 (CHEMBL3091684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50449947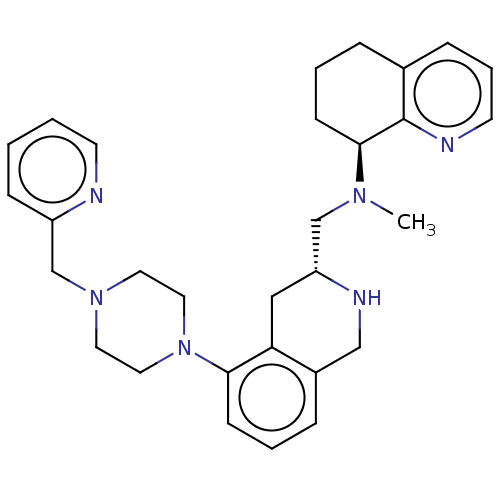 (CHEMBL4159234) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins followed b... | J Med Chem 61: 7168-7188 (2018) Article DOI: 10.1021/acs.jmedchem.8b00450 BindingDB Entry DOI: 10.7270/Q2GH9MJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315305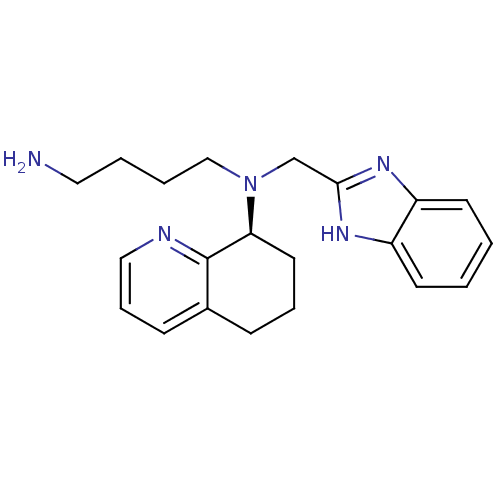 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of SDS1alpha-induced calcium flux pretreated for 25 mins followed by SDS1... | ACS Med Chem Lett 9: 446-451 (2018) Article DOI: 10.1021/acsmedchemlett.8b00030 BindingDB Entry DOI: 10.7270/Q2Z89FZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315305 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50347491 (CHEMBL1802329) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo... | J Med Chem 53: 8556-68 (2010) Article DOI: 10.1021/jm100786g BindingDB Entry DOI: 10.7270/Q2PK0GH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Mus musculus) | BDBM50270016 (CHEMBL4070320) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR4 | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50237710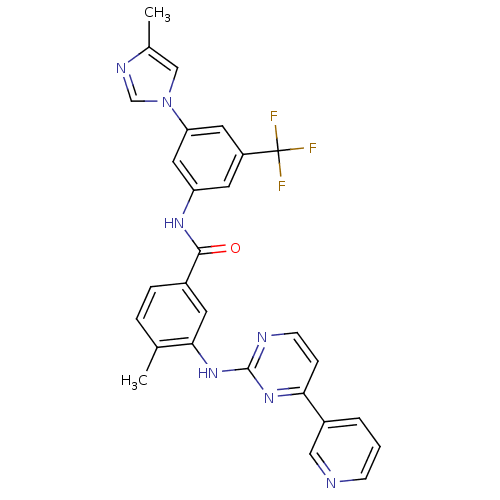 (4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human ABL1 assessed as residual activity using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125951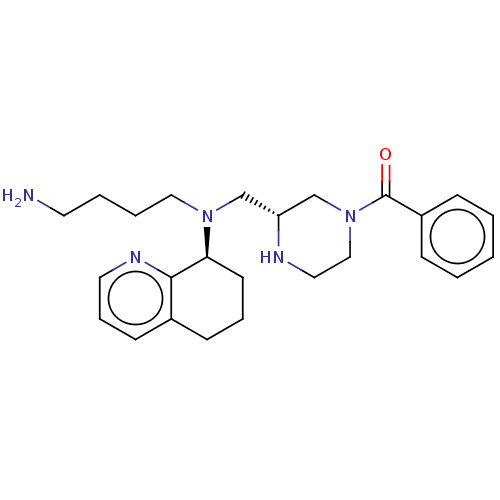 (CHEMBL3627794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50286298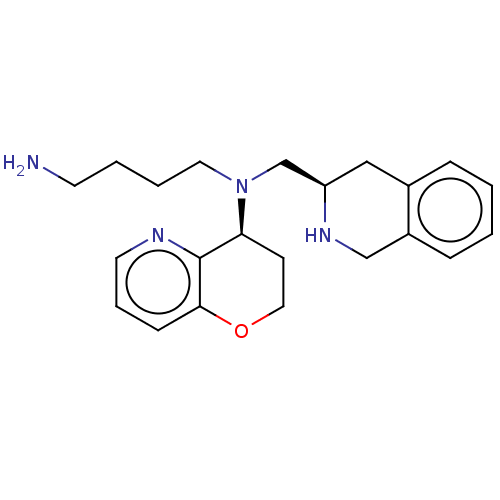 (CHEMBL4171469) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of SDF1alpha-induced calcium flux pretreated for 25 mins followed by SDF1... | ACS Med Chem Lett 9: 17-22 (2018) Article DOI: 10.1021/acsmedchemlett.7b00381 BindingDB Entry DOI: 10.7270/Q2NG4T6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of SDS1alpha-induced calcium flux pretreated for 25 mins followed by SDS1... | ACS Med Chem Lett 9: 446-451 (2018) Article DOI: 10.1021/acsmedchemlett.8b00030 BindingDB Entry DOI: 10.7270/Q2Z89FZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2230 total ) | Next | Last >> |