

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50478562 (CHEMBL502491) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Displacement of radiolabeled PDBU from PKC in rat forebrain | J Nat Prod 54: 1440-3 Article DOI: 10.1021/np50077a040 BindingDB Entry DOI: 10.7270/Q2377CGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50478561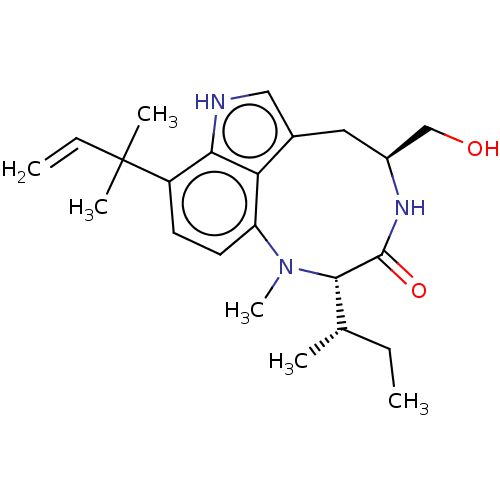 (CHEBI:69599 | Methylpendolmycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Displacement of radiolabeled PDBU from PKC in rat forebrain | J Nat Prod 54: 1440-3 Article DOI: 10.1021/np50077a040 BindingDB Entry DOI: 10.7270/Q2377CGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101892 (2-(7-Ethoxy-4-hydroxy-2,2-dioxo-2H-2lambda*6*,9-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101903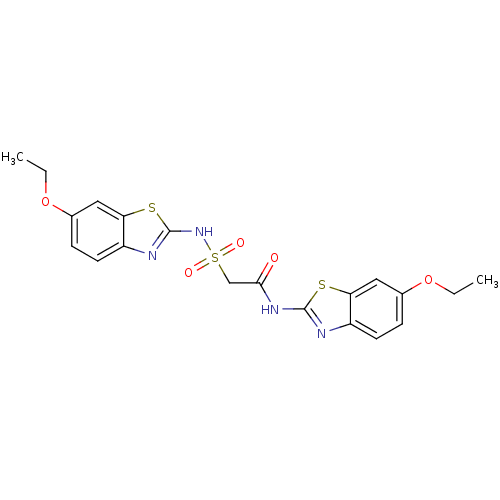 (CHEMBL50806 | N-(6-Ethoxy-benzothiazol-2-yl)-2-(6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101892 (2-(7-Ethoxy-4-hydroxy-2,2-dioxo-2H-2lambda*6*,9-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128187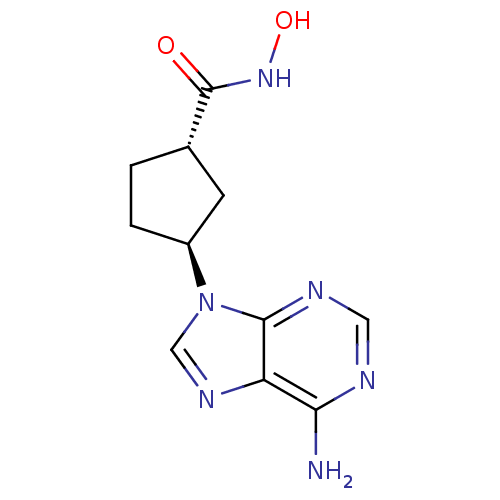 ((1R,3R)-3-(6-Amino-purin-9-yl)-cyclopentanecarboxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50292417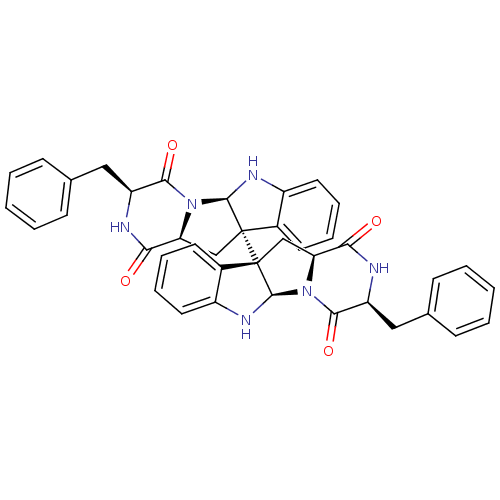 ((3S,5aR,10bR,11aS,3'S,5'R,11'R)-3,3'-Dibenzyl-2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of substance P receptor | J Nat Prod 57: 1239-1244 (1994) Article DOI: 10.1021/np50111a008 BindingDB Entry DOI: 10.7270/Q2GH9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128192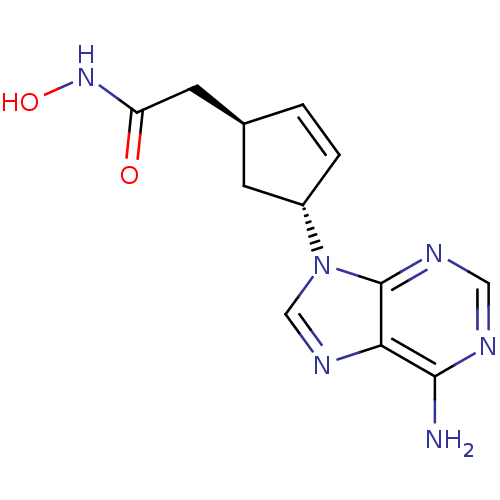 ((1S,3R)-2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128210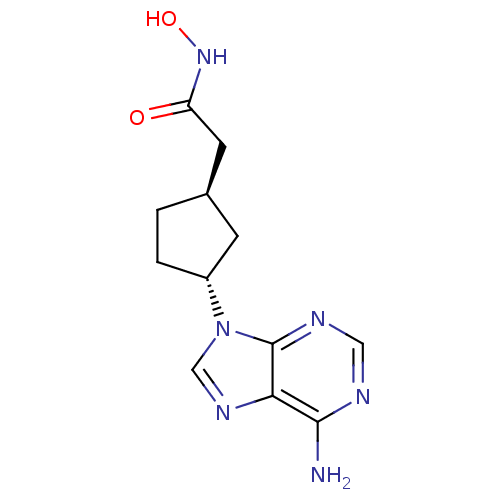 ((1R,3R)-2-[3-(6-Amino-purin-9-yl)-cyclopentyl]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101894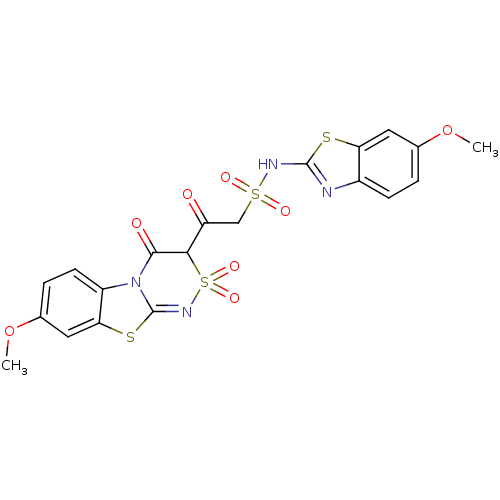 (2-(4-Hydroxy-7-methoxy-2,2-dioxo-2H-2lambda*6*,9-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101898 (2-(4-Hydroxy-7-methanesulfonyl-2,2-dioxo-2H-2lambd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101896 (3-[2-(6-Ethoxycarbonyl-benzothiazol-2-ylsulfamoyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128207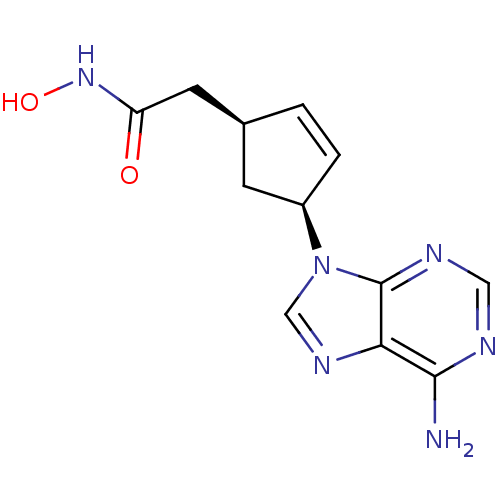 ((1S,3S)-2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128209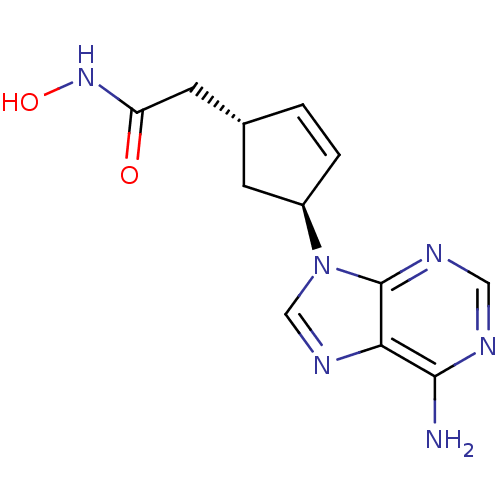 ((1R,3S)-2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128203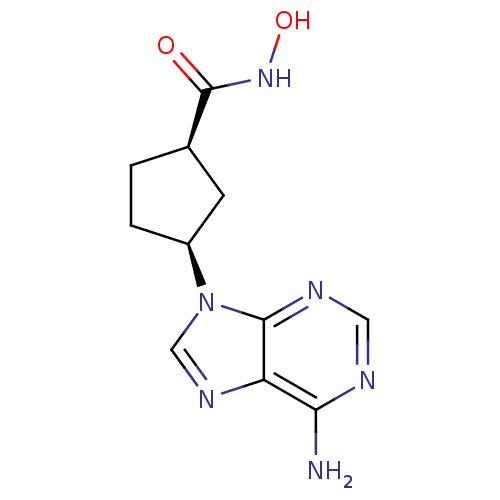 ((1R,3S)-3-(6-Amino-purin-9-yl)-cyclopentanecarboxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128199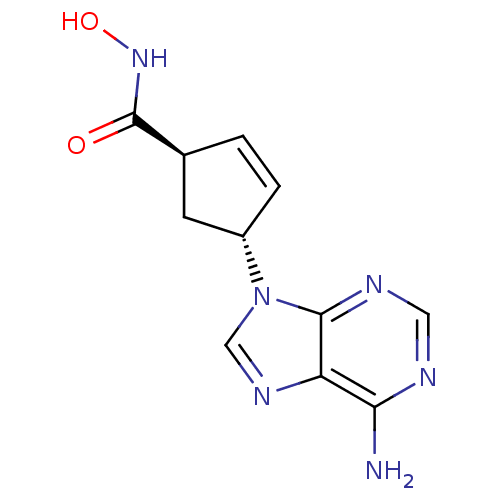 ((1S,3S)-4-(6-Amino-purin-9-yl)-cyclopent-2-enecarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50119826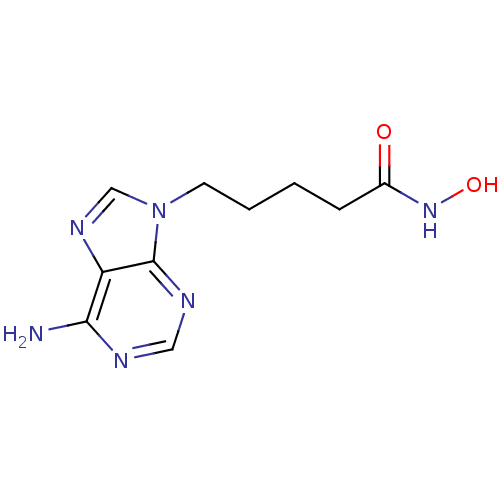 (5-(6-Amino-purin-9-yl)-pentanoic acid hydroxyamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50119832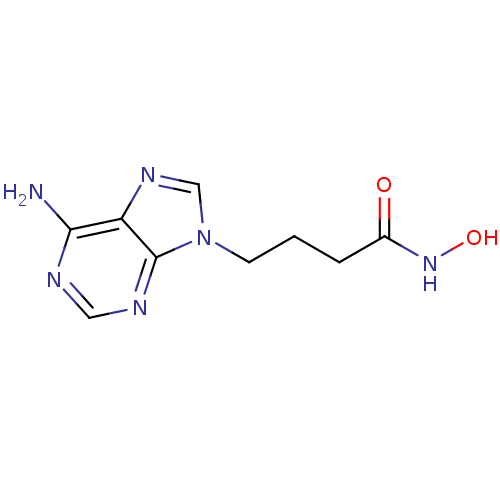 (4-(6-Amino-purin-9-yl)-N-hydroxy-butyramide | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128188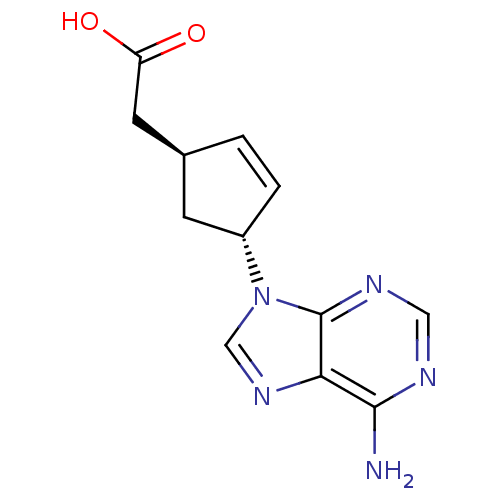 ((1S,3R)-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128206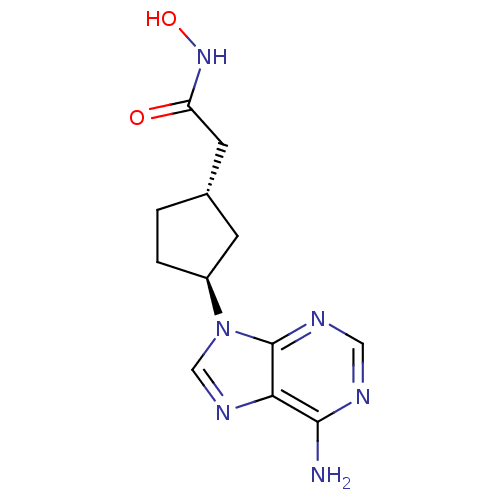 ((1S,3S)-2-[3-(6-Amino-purin-9-yl)-cyclopentyl]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128191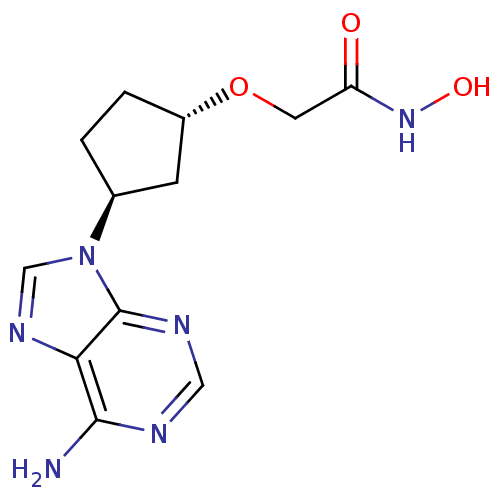 (2-[3-(6-Amino-purin-9-yl)-cyclopentyloxy]-N-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50292416 (CHEMBL501675 | Ditryptophenaline) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of substance P receptor | J Nat Prod 57: 1239-1244 (1994) Article DOI: 10.1021/np50111a008 BindingDB Entry DOI: 10.7270/Q2GH9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128205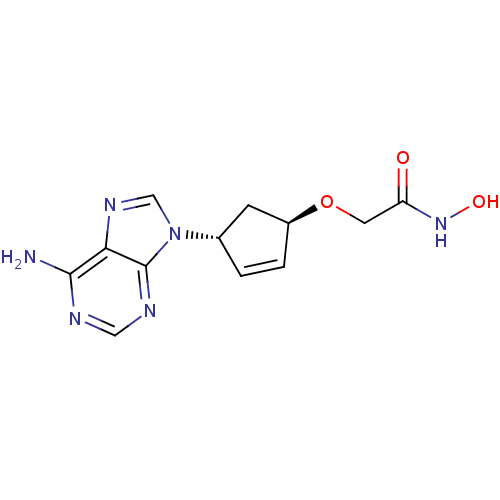 (2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyloxy]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128204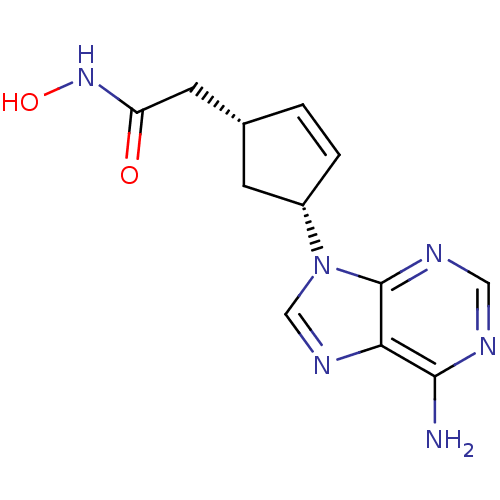 ((1R,3R)-2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101890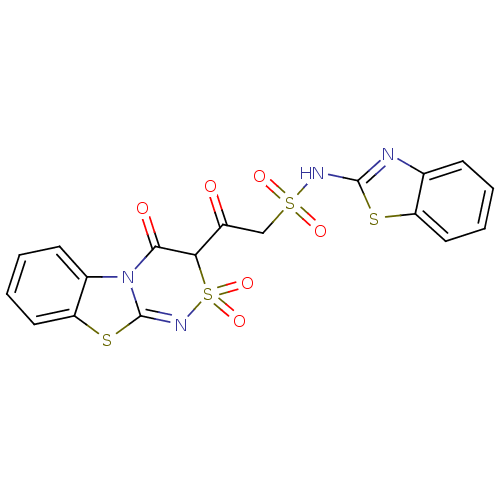 (2-(4-Hydroxy-2,2-dioxo-2H-2lambda*6*,9-dithia-1,4a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101893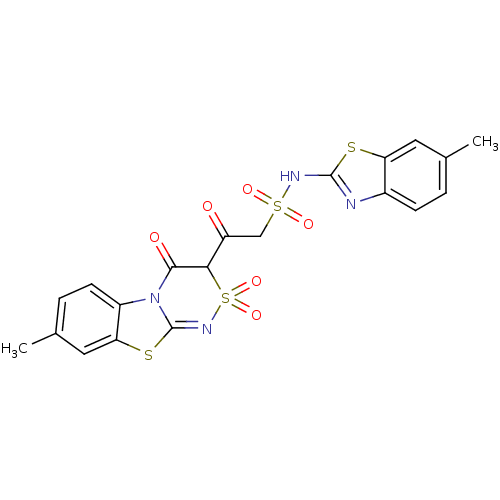 (2-(4-Hydroxy-7-methyl-2,2-dioxo-2H-2lambda*6*,9-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128202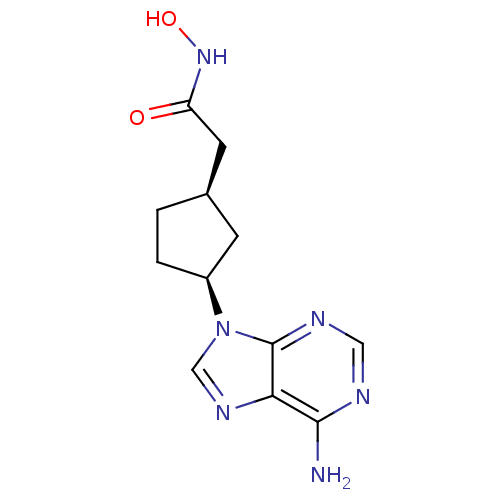 ((1R,3S)-2-[3-(6-Amino-purin-9-yl)-cyclopentyl]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128195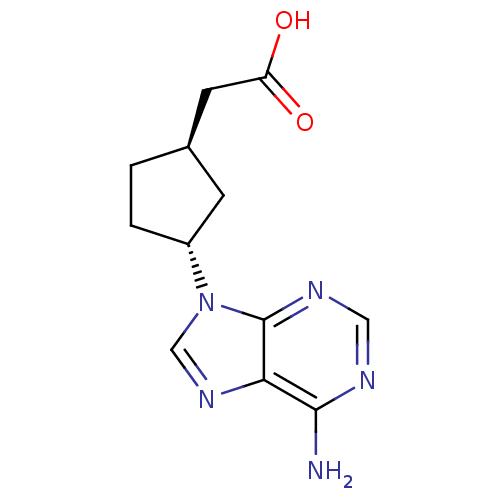 ((1R,3R)-[3-(6-Amino-purin-9-yl)-cyclopentyl]-aceti...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101900 (2-(4-Hydroxy-7-nitro-2,2-dioxo-2H-2lambda*6*,9-dit...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101895 (2-(7-Chloro-4-hydroxy-2,2-dioxo-2H-2lambda*6*,9-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50119842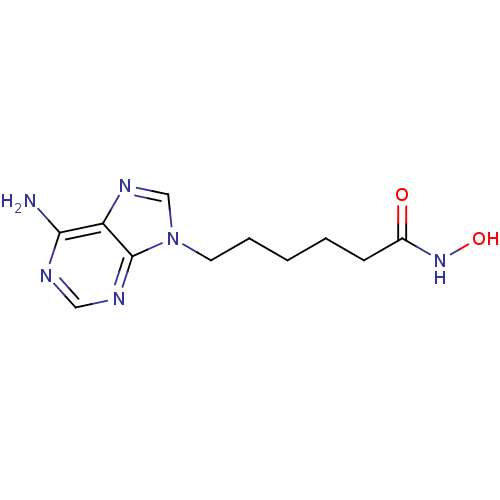 (6-(6-Amino-purin-9-yl)-hexanoic acid hydroxyamide ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101897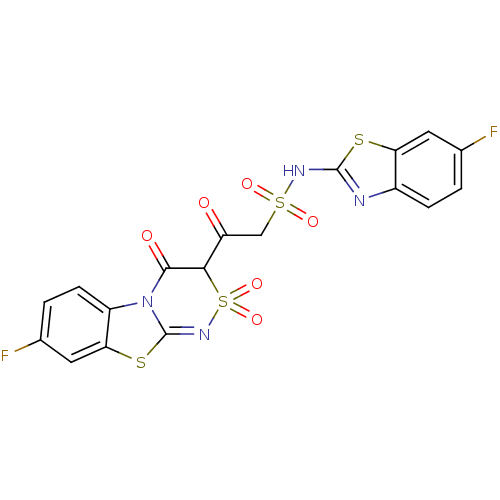 (2-(7-Fluoro-4-hydroxy-2,2-dioxo-2H-2lambda*6*,9-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101899 (2-(4-Hydroxy-2,2-dioxo-7-phenoxy-2H-2lambda*6*,9-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101902 (2-(4-Hydroxy-2,2-dioxo-7-phenyl-2H-2lambda*6*,9-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128197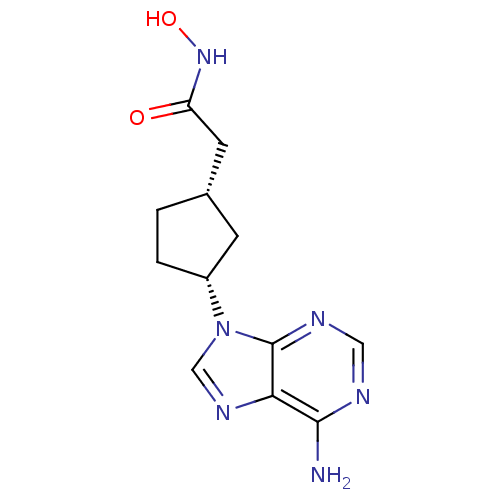 ((1S,3R)-2-[3-(6-Amino-purin-9-yl)-cyclopentyl]-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128201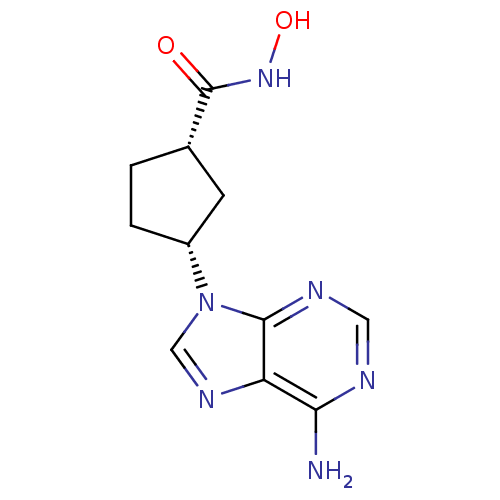 ((1S,3R)-3-(6-Amino-purin-9-yl)-cyclopentanecarboxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128190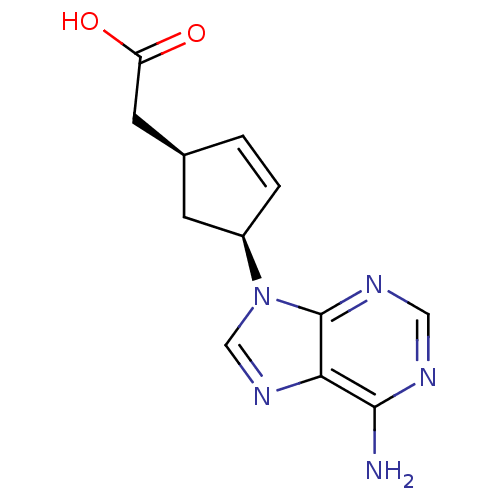 ((1S,3S)-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101891 (5-Methoxy-2,2-dioxo-2H-2lambda*6*,9-dithia-1,4a-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128186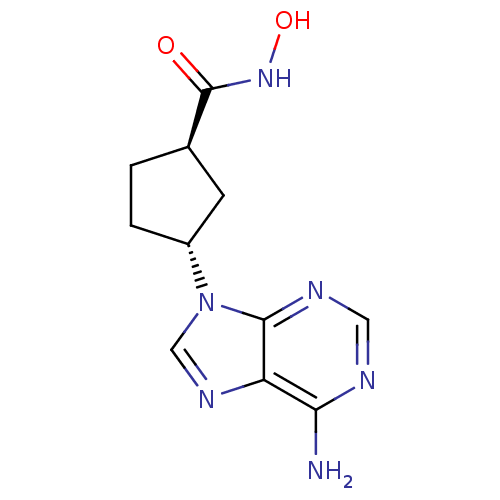 ((1S,3S)-3-(6-Amino-purin-9-yl)-cyclopentanecarboxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128189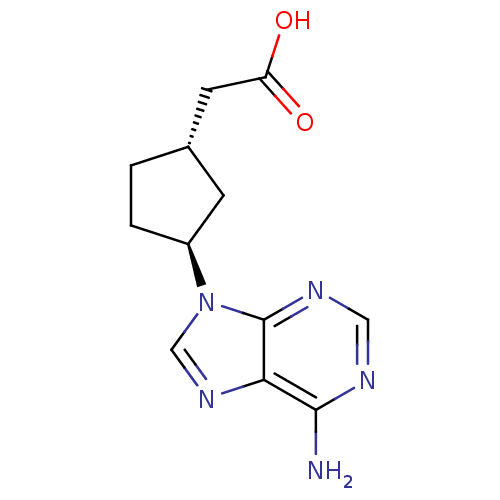 ((1S,3S)-[3-(6-Amino-purin-9-yl)-cyclopentyl]-aceti...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128194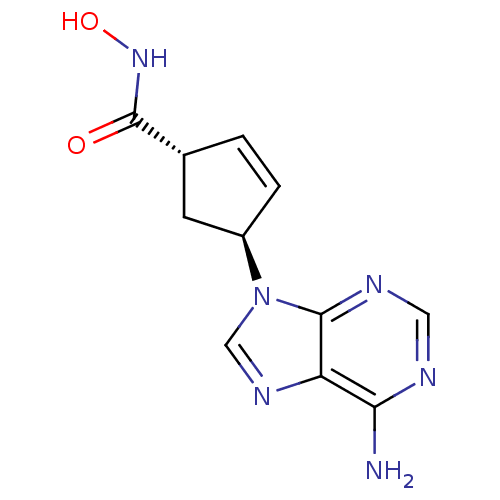 ((1R,3R)-4-(6-Amino-purin-9-yl)-cyclopent-2-enecarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101901 (2,2-Dioxo-7-trifluoromethoxy-2H-2lambda*6*,9-dithi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50101889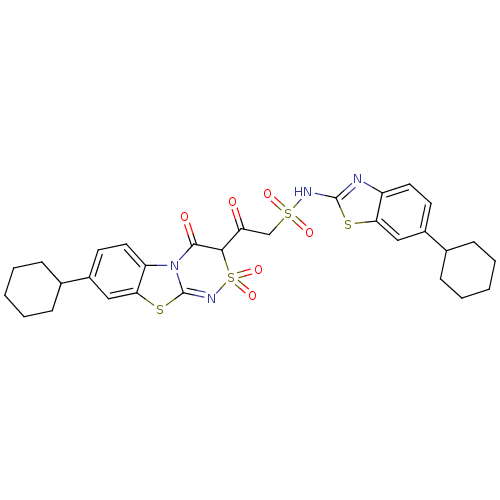 (2-(7-Cyclohexyl-4-hydroxy-2,2-dioxo-2H-2lambda*6*,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Ability to displace [3H]-2-MeS-ADP from human Purinergic receptor P2Y12 | Bioorg Med Chem Lett 11: 1805-8 (2001) BindingDB Entry DOI: 10.7270/Q2VT1RC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128211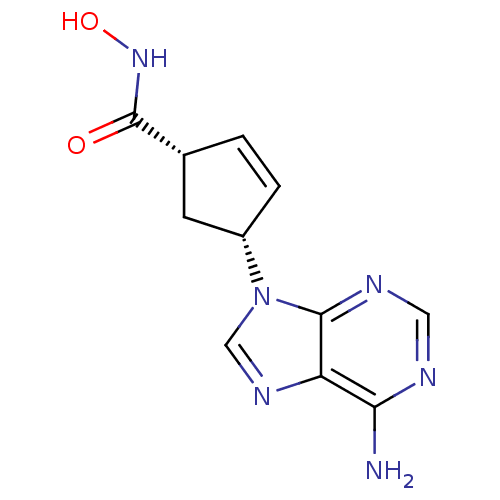 ((1S,3R)-4-(6-Amino-purin-9-yl)-cyclopent-2-enecarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128200 ((1S,3R)-[3-(6-Amino-purin-9-yl)-cyclopentyl]-aceti...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128198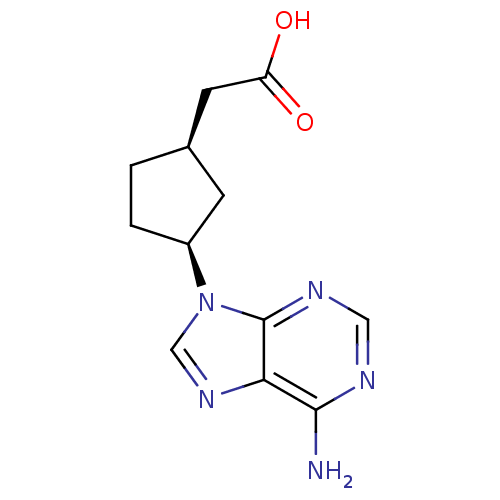 ((1R,3S)-[3-(6-Amino-purin-9-yl)-cyclopentyl]-aceti...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128196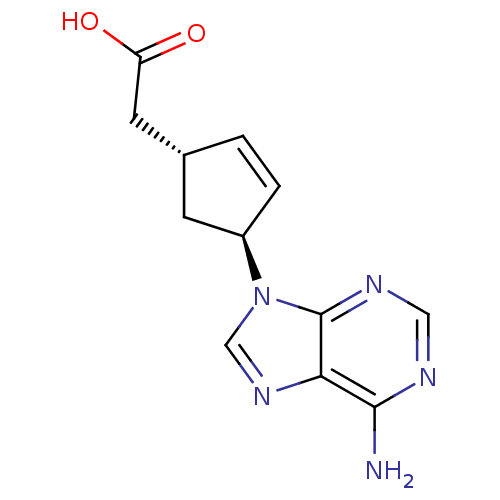 ((1R,3S)-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128193 ((1R,3S)-4-(6-Amino-purin-9-yl)-cyclopent-2-enecarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Homo sapiens (Human)) | BDBM50128208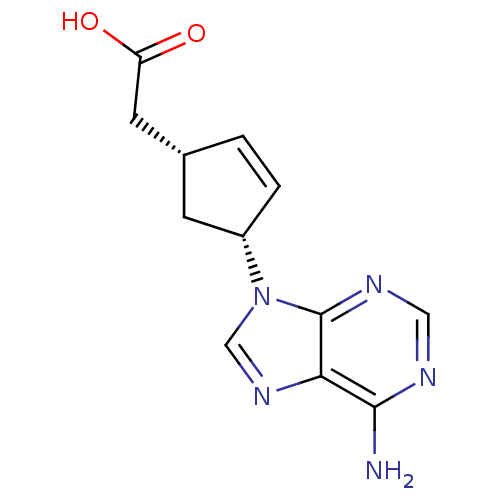 ((1R,3R)-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. | J Med Chem 46: 2177-86 (2003) Article DOI: 10.1021/jm0205604 BindingDB Entry DOI: 10.7270/Q2M32V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||