 Found 269 hits with Last Name = 'sem' and Initial = 'ds'
Found 269 hits with Last Name = 'sem' and Initial = 'ds' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity protein phosphatase 5 [180-384]
(Homo sapiens (Human)) | BDBM50336799
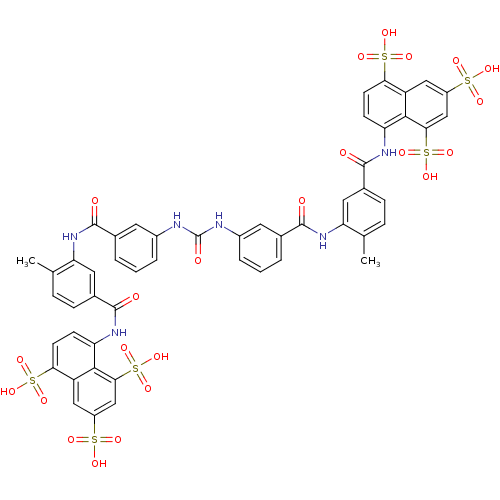
(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | -43.4 | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Texas Wesleyan University
| Assay Description
For the 96-well plate validation assay, sodium orthovanadate (Sigma Aldrich) was utilized as a positive control for inhibition [Swarup et al., Bioche... |
BMC Biochem 16: 19 (2015)
Article DOI: 10.1186/s12858-015-0048-3
BindingDB Entry DOI: 10.7270/Q26972FW |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59098
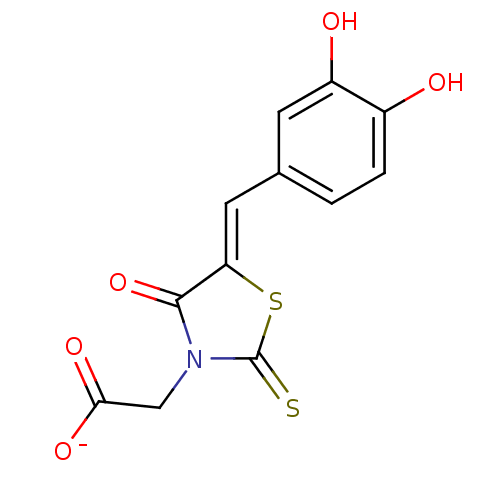
(Bi-ligand, 1)Show InChI InChI=1S/C12H9NO5S2/c14-7-2-1-6(3-8(7)15)4-9-11(18)13(5-10(16)17)12(19)20-9/h1-4,14-15H,5H2,(H,16,17)/p-1/b9-4- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59099
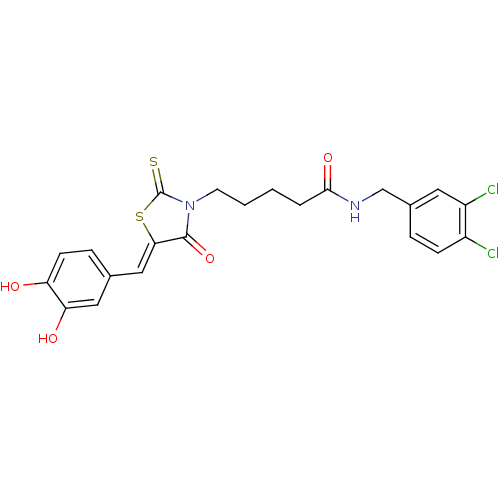
(Bi-ligand, 2)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CCCCC(=O)NCc3ccc(Cl)c(Cl)c3)C2=O)cc1O Show InChI InChI=1S/C22H20Cl2N2O4S2/c23-15-6-4-14(9-16(15)24)12-25-20(29)3-1-2-8-26-21(30)19(32-22(26)31)11-13-5-7-17(27)18(28)10-13/h4-7,9-11,27-28H,1-3,8,12H2,(H,25,29)/b19-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59101
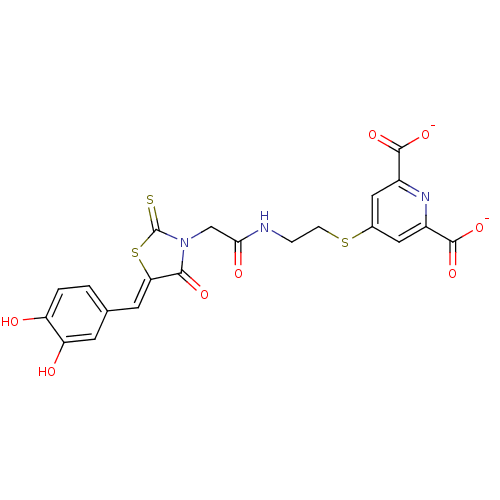
(Bi-ligand, 4)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CC(=O)NCCSc3cc(nc(c3)C([O-])=O)C([O-])=O)C2=O)cc1O Show InChI InChI=1S/C21H17N3O8S3/c25-14-2-1-10(5-15(14)26)6-16-18(28)24(21(33)35-16)9-17(27)22-3-4-34-11-7-12(19(29)30)23-13(8-11)20(31)32/h1-2,5-8,25-26H,3-4,9H2,(H,22,27)(H,29,30)(H,31,32)/p-2/b16-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM59100
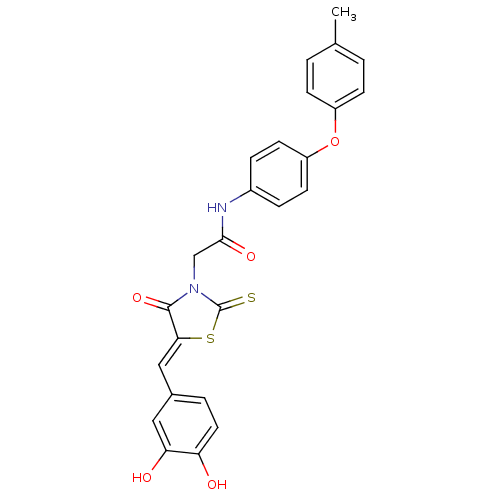
(Bi-ligand, 3)Show SMILES Cc1ccc(Oc2ccc(NC(=O)CN3C(=S)S\C(=C/c4ccc(O)c(O)c4)C3=O)cc2)cc1 Show InChI InChI=1S/C25H20N2O5S2/c1-15-2-7-18(8-3-15)32-19-9-5-17(6-10-19)26-23(30)14-27-24(31)22(34-25(27)33)13-16-4-11-20(28)21(29)12-16/h2-13,28-29H,14H2,1H3,(H,26,30)/b22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 202 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59101

(Bi-ligand, 4)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CC(=O)NCCSc3cc(nc(c3)C([O-])=O)C([O-])=O)C2=O)cc1O Show InChI InChI=1S/C21H17N3O8S3/c25-14-2-1-10(5-15(14)26)6-16-18(28)24(21(33)35-16)9-17(27)22-3-4-34-11-7-12(19(29)30)23-13(8-11)20(31)32/h1-2,5-8,25-26H,3-4,9H2,(H,22,27)(H,29,30)(H,31,32)/p-2/b16-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM59101

(Bi-ligand, 4)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CC(=O)NCCSc3cc(nc(c3)C([O-])=O)C([O-])=O)C2=O)cc1O Show InChI InChI=1S/C21H17N3O8S3/c25-14-2-1-10(5-15(14)26)6-16-18(28)24(21(33)35-16)9-17(27)22-3-4-34-11-7-12(19(29)30)23-13(8-11)20(31)32/h1-2,5-8,25-26H,3-4,9H2,(H,22,27)(H,29,30)(H,31,32)/p-2/b16-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM59099

(Bi-ligand, 2)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CCCCC(=O)NCc3ccc(Cl)c(Cl)c3)C2=O)cc1O Show InChI InChI=1S/C22H20Cl2N2O4S2/c23-15-6-4-14(9-16(15)24)12-25-20(29)3-1-2-8-26-21(30)19(32-22(26)31)11-13-5-7-17(27)18(28)10-13/h4-7,9-11,27-28H,1-3,8,12H2,(H,25,29)/b19-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59100

(Bi-ligand, 3)Show SMILES Cc1ccc(Oc2ccc(NC(=O)CN3C(=S)S\C(=C/c4ccc(O)c(O)c4)C3=O)cc2)cc1 Show InChI InChI=1S/C25H20N2O5S2/c1-15-2-7-18(8-3-15)32-19-9-5-17(6-10-19)26-23(30)14-27-24(31)22(34-25(27)33)13-16-4-11-20(28)21(29)12-16/h2-13,28-29H,14H2,1H3,(H,26,30)/b22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59100

(Bi-ligand, 3)Show SMILES Cc1ccc(Oc2ccc(NC(=O)CN3C(=S)S\C(=C/c4ccc(O)c(O)c4)C3=O)cc2)cc1 Show InChI InChI=1S/C25H20N2O5S2/c1-15-2-7-18(8-3-15)32-19-9-5-17(6-10-19)26-23(30)14-27-24(31)22(34-25(27)33)13-16-4-11-20(28)21(29)12-16/h2-13,28-29H,14H2,1H3,(H,26,30)/b22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59099

(Bi-ligand, 2)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CCCCC(=O)NCc3ccc(Cl)c(Cl)c3)C2=O)cc1O Show InChI InChI=1S/C22H20Cl2N2O4S2/c23-15-6-4-14(9-16(15)24)12-25-20(29)3-1-2-8-26-21(30)19(32-22(26)31)11-13-5-7-17(27)18(28)10-13/h4-7,9-11,27-28H,1-3,8,12H2,(H,25,29)/b19-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM59098

(Bi-ligand, 1)Show InChI InChI=1S/C12H9NO5S2/c14-7-2-1-6(3-8(7)15)4-9-11(18)13(5-10(16)17)12(19)20-9/h1-4,14-15H,5H2,(H,16,17)/p-1/b9-4- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59098

(Bi-ligand, 1)Show InChI InChI=1S/C12H9NO5S2/c14-7-2-1-6(3-8(7)15)4-9-11(18)13(5-10(16)17)12(19)20-9/h1-4,14-15H,5H2,(H,16,17)/p-1/b9-4- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Antagonist activity at ERbeta (unknown origin) by cell-based assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0953 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain fused VDR (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed as beta-lac... |
Eur J Med Chem 157: 791-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.006
BindingDB Entry DOI: 10.7270/Q2K64MS7 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM398047
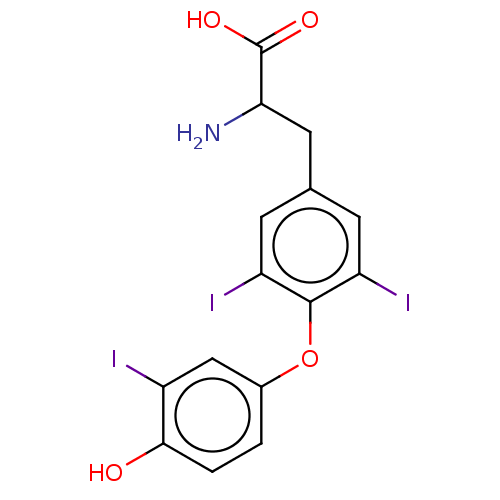
(US10322118, Entry 5)Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain fused TRbeta receptor (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed... |
Eur J Med Chem 157: 791-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.006
BindingDB Entry DOI: 10.7270/Q2K64MS7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain fused ERalpha (unknown origin) ligand binding domain expressed in UAS-bla GripTite 293 cells assessed as ... |
Eur J Med Chem 157: 791-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.006
BindingDB Entry DOI: 10.7270/Q2K64MS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18660

((10S,11S,14S,15S)-14,15-dimethyl-14-propanoyltetra...)Show SMILES [H][C@@]12CC[C@](C)(C(=O)CC)[C@@]1(C)CCC1=C3CCC(=O)C=C3CC[C@@]21[H] |r,c:15,21| Show InChI InChI=1S/C22H30O2/c1-4-20(24)22(3)12-10-19-18-7-5-14-13-15(23)6-8-16(14)17(18)9-11-21(19,22)2/h13,18-19H,4-12H2,1-3H3/t18-,19+,21+,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.236 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain fused PR (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed as beta-lact... |
Eur J Med Chem 157: 791-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.006
BindingDB Entry DOI: 10.7270/Q2K64MS7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128906
BindingDB Entry DOI: 10.7270/Q2S186FF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain fused androgen receptor (unknown origin) ligand binding domain expressed in UAS-bla GripTite 293 cells as... |
Eur J Med Chem 157: 791-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.006
BindingDB Entry DOI: 10.7270/Q2K64MS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM19214
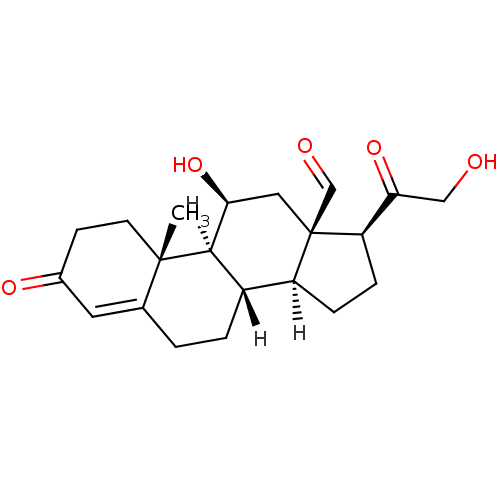
((1S,2R,10S,11S,14S,15R,17S)-17-hydroxy-14-(2-hydro...)Show SMILES [H][C@@]12CC[C@H](C(=O)CO)[C@]1(C[C@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C)C=O |t:21| Show InChI InChI=1S/C21H28O5/c1-20-7-6-13(24)8-12(20)2-3-14-15-4-5-16(18(26)10-22)21(15,11-23)9-17(25)19(14)20/h8,11,14-17,19,22,25H,2-7,9-10H2,1H3/t14-,15-,16+,17-,19+,20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.305 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain fused MR (unknown origin) ligand binding domain expressed in UAS-bla H cells assessed as beta-lactamase t... |
Eur J Med Chem 157: 791-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.006
BindingDB Entry DOI: 10.7270/Q2K64MS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.579 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain fused ERbeta (unknown origin) ligand binding domain expressed in UAS-bla GripTite 293 cells assessed as b... |
Eur J Med Chem 157: 791-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.006
BindingDB Entry DOI: 10.7270/Q2K64MS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50589452
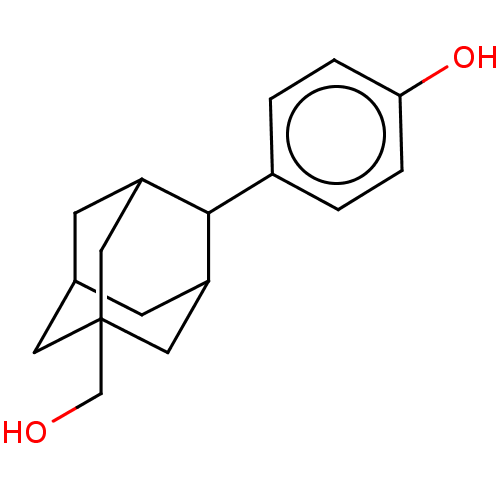
(CHEMBL5184751)Show SMILES OCC12CC3CC(C1)C(C(C3)C2)c1ccc(O)cc1 |TLB:1:2:5:8.9.10,12:8:5:2.3.7,THB:11:9:5:2.3.7,11:2:5:8.9.10,3:4:8:11.2.7,3:2:8:4.5.10,(4.67,4.29,;3.15,4.05,;2.78,2.55,;4.11,1.87,;3.97,.53,;3.05,-.72,;1.63,-.15,;1.81,1.56,;.11,-.41,;1.02,.84,;2.45,.27,;1.15,2.08,;-1.08,-1.38,;-.84,-2.9,;-2.03,-3.87,;-3.47,-3.32,;-4.67,-4.29,;-3.72,-1.81,;-2.53,-.83,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128906
BindingDB Entry DOI: 10.7270/Q2S186FF |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha (unknown origin) by cell-based assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128906
BindingDB Entry DOI: 10.7270/Q2S186FF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain fused GR (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed as beta-lact... |
Eur J Med Chem 157: 791-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.006
BindingDB Entry DOI: 10.7270/Q2K64MS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-tagged estrogen receptor alpha ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay |
J Med Chem 61: 4720-4738 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01601
BindingDB Entry DOI: 10.7270/Q2TH8R3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-tagged estrogen receptor beta ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay |
J Med Chem 61: 4720-4738 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01601
BindingDB Entry DOI: 10.7270/Q2TH8R3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50589454
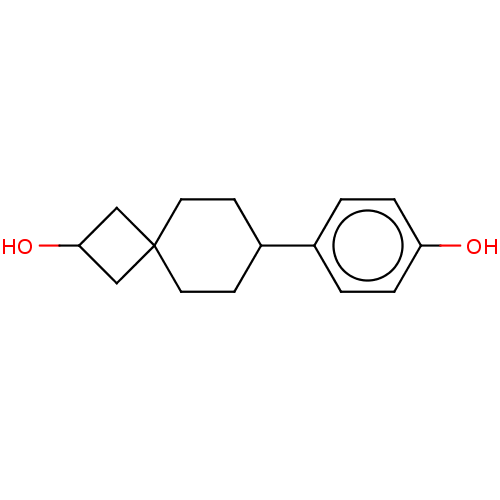
(CHEMBL5186437) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128906
BindingDB Entry DOI: 10.7270/Q2S186FF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50466084

(CHEMBL3099433)Show InChI InChI=1S/C14H20O2/c15-10-11-2-1-3-12(5-4-11)13-6-8-14(16)9-7-13/h6-9,11-12,15-16H,1-5,10H2/t11-,12+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50494962

(CHEMBL3099432)Show SMILES [H][C@@]12CC=C(CO)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r,t:3| Show InChI InChI=1S/C19H24O2/c1-19-9-8-16-15-6-4-14(21)10-12(15)2-5-17(16)18(19)7-3-13(19)11-20/h3-4,6,10,16-18,20-21H,2,5,7-9,11H2,1H3/t16-,17-,18+,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50494959

(CHEMBL3099429)Show SMILES [H][C@@]12CC[C@H](C(=O)OCCC)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C22H30O3/c1-3-12-25-21(24)20-9-8-19-18-6-4-14-13-15(23)5-7-16(14)17(18)10-11-22(19,20)2/h5,7,13,17-20,23H,3-4,6,8-12H2,1-2H3/t17-,18-,19+,20-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50085041
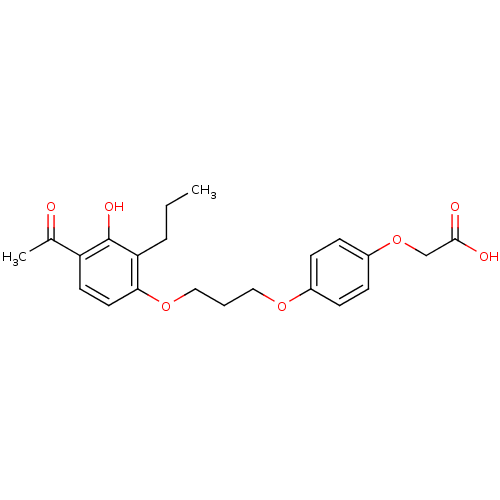
(2-(4-(3-(4-acetyl-3-hydroxy-2-propylphenoxy)propox...)Show SMILES CCCc1c(O)c(ccc1OCCCOc1ccc(OCC(O)=O)cc1)C(C)=O Show InChI InChI=1S/C22H26O7/c1-3-5-19-20(11-10-18(15(2)23)22(19)26)28-13-4-12-27-16-6-8-17(9-7-16)29-14-21(24)25/h6-11,26H,3-5,12-14H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4 DNA-binding domain fused PPARdelta receptor (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells asses... |
Eur J Med Chem 157: 791-804 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.006
BindingDB Entry DOI: 10.7270/Q2K64MS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50589454

(CHEMBL5186437) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128906
BindingDB Entry DOI: 10.7270/Q2S186FF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50494960
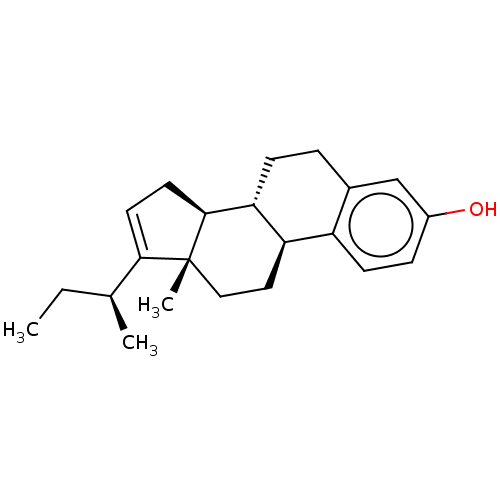
(CHEMBL3099430 | US10570077, Compound 7)Show SMILES [H][C@@]12CC=C([C@@H](C)CC)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r,t:3| Show InChI InChI=1S/C22H30O/c1-4-14(2)20-9-10-21-19-7-5-15-13-16(23)6-8-17(15)18(19)11-12-22(20,21)3/h6,8-9,13-14,18-19,21,23H,4-5,7,10-12H2,1-3H3/t14-,18+,19+,21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Antagonist activity at ERbeta (unknown origin) assessed as inhibition of E2-induced receptor activation after 22 hrs by cell-based luciferase reporte... |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50589452

(CHEMBL5184751)Show SMILES OCC12CC3CC(C1)C(C(C3)C2)c1ccc(O)cc1 |TLB:1:2:5:8.9.10,12:8:5:2.3.7,THB:11:9:5:2.3.7,11:2:5:8.9.10,3:4:8:11.2.7,3:2:8:4.5.10,(4.67,4.29,;3.15,4.05,;2.78,2.55,;4.11,1.87,;3.97,.53,;3.05,-.72,;1.63,-.15,;1.81,1.56,;.11,-.41,;1.02,.84,;2.45,.27,;1.15,2.08,;-1.08,-1.38,;-.84,-2.9,;-2.03,-3.87,;-3.47,-3.32,;-4.67,-4.29,;-3.72,-1.81,;-2.53,-.83,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128906
BindingDB Entry DOI: 10.7270/Q2S186FF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50494960

(CHEMBL3099430 | US10570077, Compound 7)Show SMILES [H][C@@]12CC=C([C@@H](C)CC)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r,t:3| Show InChI InChI=1S/C22H30O/c1-4-14(2)20-9-10-21-19-7-5-15-13-16(23)6-8-17(15)18(19)11-12-22(20,21)3/h6,8-9,13-14,18-19,21,23H,4-5,7,10-12H2,1-3H3/t14-,18+,19+,21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50494959

(CHEMBL3099429)Show SMILES [H][C@@]12CC[C@H](C(=O)OCCC)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C22H30O3/c1-3-12-25-21(24)20-9-8-19-18-6-4-14-13-15(23)5-7-16(14)17(18)10-11-22(19,20)2/h5,7,13,17-20,23H,3-4,6,8-12H2,1-2H3/t17-,18-,19+,20-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50494961
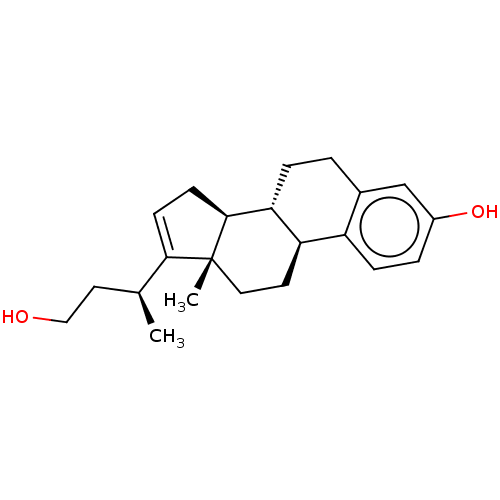
(CHEMBL3099428 | US10570077, Compound 11)Show SMILES [H][C@@]12CC=C([C@@H](C)CCO)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r,t:3| Show InChI InChI=1S/C22H30O2/c1-14(10-12-23)20-7-8-21-19-5-3-15-13-16(24)4-6-17(15)18(19)9-11-22(20,21)2/h4,6-7,13-14,18-19,21,23-24H,3,5,8-12H2,1-2H3/t14-,18+,19+,21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50494963

(CHEMBL3099431 | US10570077, Compound 13)Show SMILES [H][C@@]12CCC3(OC(C)C[C@@H]3C)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C23H32O2/c1-14-12-15(2)25-23(14)11-9-21-20-6-4-16-13-17(24)5-7-18(16)19(20)8-10-22(21,23)3/h5,7,13-15,19-21,24H,4,6,8-12H2,1-3H3/t14-,15?,19+,20+,21-,22-,23?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50466084

(CHEMBL3099433)Show InChI InChI=1S/C14H20O2/c15-10-11-2-1-3-12(5-4-11)13-6-8-14(16)9-7-13/h6-9,11-12,15-16H,1-5,10H2/t11-,12+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Antagonist activity at ERbeta (unknown origin) assessed as inhibition of E2-induced receptor activation after 22 hrs by cell-based luciferase reporte... |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50494962

(CHEMBL3099432)Show SMILES [H][C@@]12CC=C(CO)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r,t:3| Show InChI InChI=1S/C19H24O2/c1-19-9-8-16-15-6-4-14(21)10-12(15)2-5-17(16)18(19)7-3-13(19)11-20/h3-4,6,10,16-18,20-21H,2,5,7-9,11H2,1H3/t16-,17-,18+,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50517935
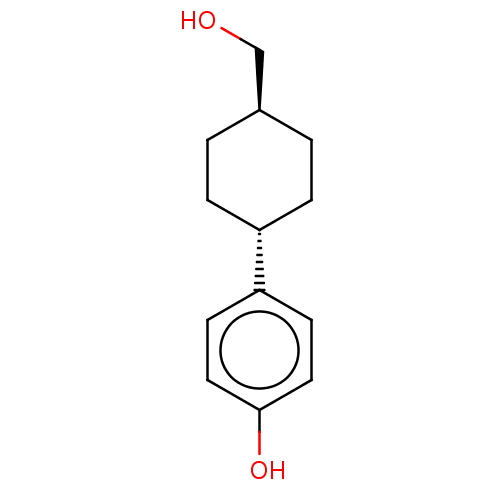
(CHEMBL4526434)Show SMILES OC[C@H]1CC[C@@H](CC1)c1ccc(O)cc1 |r,wU:5.8,wD:2.1,(30.01,-26.44,;29.24,-27.77,;27.7,-27.77,;26.93,-26.43,;25.39,-26.43,;24.62,-27.76,;25.39,-29.1,;26.93,-29.1,;23.08,-27.76,;22.32,-26.42,;20.78,-26.42,;20.01,-27.76,;18.47,-27.76,;20.79,-29.09,;22.32,-29.09,)| Show InChI InChI=1S/C13H18O2/c14-9-10-1-3-11(4-2-10)12-5-7-13(15)8-6-12/h5-8,10-11,14-15H,1-4,9H2/t10-,11- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin
Curated by ChEMBL
| Assay Description
Agonist activity at human GST-tagged estrogen receptor beta ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay |
J Med Chem 61: 4720-4738 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01601
BindingDB Entry DOI: 10.7270/Q2TH8R3Q |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50494961

(CHEMBL3099428 | US10570077, Compound 11)Show SMILES [H][C@@]12CC=C([C@@H](C)CCO)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r,t:3| Show InChI InChI=1S/C22H30O2/c1-14(10-12-23)20-7-8-21-19-5-3-15-13-16(24)4-6-17(15)18(19)9-11-22(20,21)2/h4,6-7,13-14,18-19,21,23-24H,3,5,8-12H2,1-2H3/t14-,18+,19+,21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Antagonist activity at ERbeta (unknown origin) assessed as inhibition of E2-induced receptor activation after 22 hrs by cell-based luciferase reporte... |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50494963

(CHEMBL3099431 | US10570077, Compound 13)Show SMILES [H][C@@]12CCC3(OC(C)C[C@@H]3C)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C23H32O2/c1-14-12-15(2)25-23(14)11-9-21-20-6-4-16-13-17(24)5-7-18(16)19(20)8-10-22(21,23)3/h5,7,13-15,19-21,24H,4,6,8-12H2,1-3H3/t14-,15?,19+,20+,21-,22-,23?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University
Curated by ChEMBL
| Assay Description
Agonist activity at ERalpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay |
Bioorg Med Chem 22: 303-10 (2014)
Article DOI: 10.1016/j.bmc.2013.11.024
BindingDB Entry DOI: 10.7270/Q2FF3WBH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM231697
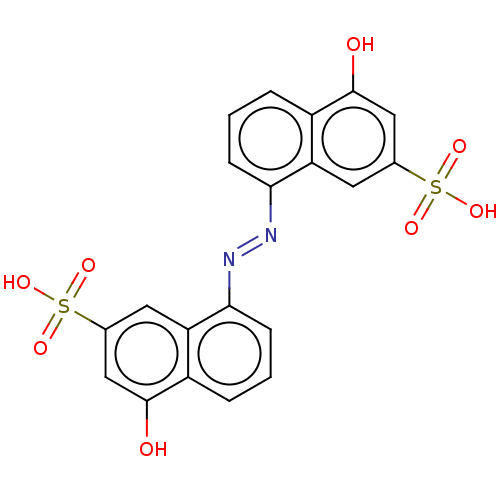
(RR601)Show SMILES Oc1cc(cc2c(cccc12)\N=N\c1cccc2c(O)cc(cc12)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C20H14N2O8S2/c23-19-9-11(31(25,26)27)7-15-13(19)3-1-5-17(15)21-22-18-6-2-4-14-16(18)8-12(10-20(14)24)32(28,29)30/h1-10,23-24H,(H,25,26,27)(H,28,29,30)/b22-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Concordia University of Wisconsin
| Assay Description
Assays with and without inhibitor were performed in Corning 96-well clear bottom plates having a nonbinding surface, with a total assay volume of 200... |
BMC Biochem 18: 10 (2017)
Article DOI: 10.1186/s12858-017-0083-3
BindingDB Entry DOI: 10.7270/Q2JW8CSF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50589453
(CHEMBL5187139) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128906
BindingDB Entry DOI: 10.7270/Q2S186FF |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 5
(Homo sapiens (Human)) | BDBM231694

(1-Amin-5-Naphthol-7-Sulfonic acid | NCI2602 | RR53...)Show InChI InChI=1S/C10H9NO4S/c11-9-3-1-2-7-8(9)4-6(5-10(7)12)16(13,14)15/h1-5,12H,11H2,(H,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University of Wisconsin
| Assay Description
This assay was done with full-length protein (containing both domains), andusing pERK as substrate. |
BMC Biochem 18: 10 (2017)
Article DOI: 10.1186/s12858-017-0083-3
BindingDB Entry DOI: 10.7270/Q2JW8CSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM231697

(RR601)Show SMILES Oc1cc(cc2c(cccc12)\N=N\c1cccc2c(O)cc(cc12)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C20H14N2O8S2/c23-19-9-11(31(25,26)27)7-15-13(19)3-1-5-17(15)21-22-18-6-2-4-14-16(18)8-12(10-20(14)24)32(28,29)30/h1-10,23-24H,(H,25,26,27)(H,28,29,30)/b22-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Concordia University of Wisconsin
| Assay Description
Assays with and without inhibitor were performed in Corning 96-well clear bottom plates having a nonbinding surface, with a total assay volume of 200... |
BMC Biochem 18: 10 (2017)
Article DOI: 10.1186/s12858-017-0083-3
BindingDB Entry DOI: 10.7270/Q2JW8CSF |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50589453

(CHEMBL5187139) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128906
BindingDB Entry DOI: 10.7270/Q2S186FF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data