

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5052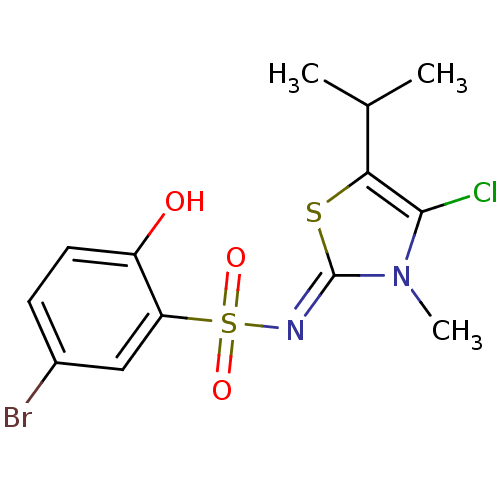 (5-bromo-N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183273 ((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase strand transfer activity | J Med Chem 49: 1506-8 (2006) Article DOI: 10.1021/jm0600139 BindingDB Entry DOI: 10.7270/Q2H131NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102044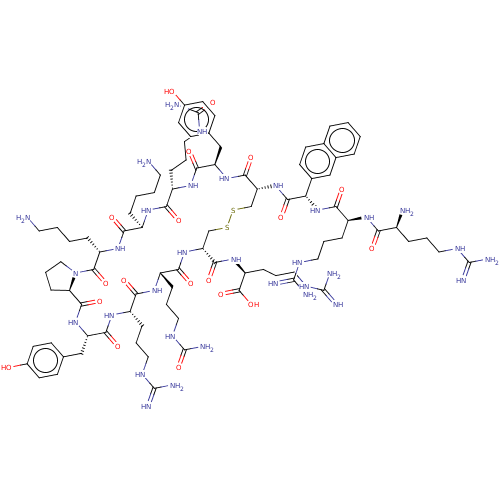 (CHEMBL2373002 | Derivative of T140 peptide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183275 ((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase strand transfer activity | J Med Chem 49: 1506-8 (2006) Article DOI: 10.1021/jm0600139 BindingDB Entry DOI: 10.7270/Q2H131NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5054 (5-Chloro-N-(4-chloro-5-isopropyl-3-methyl-1,3-thia...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183274 (6-(3-Chloro-2-fluorobenzyl)-1-(2-hydroxyethyl)-7-m...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase strand transfer activity | J Med Chem 49: 1506-8 (2006) Article DOI: 10.1021/jm0600139 BindingDB Entry DOI: 10.7270/Q2H131NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5051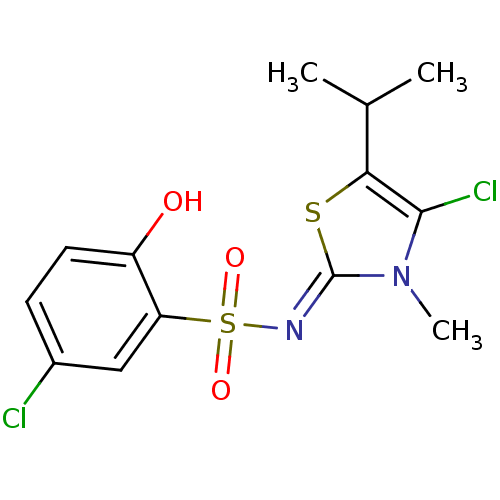 (5-Chloro-N-(4-chloro-5-isopropyl-3-methyl-13-thiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5055 (N-(4-Chloro-5-isopropyl-3-methyl-1,3-thiazol-2(3H)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50096735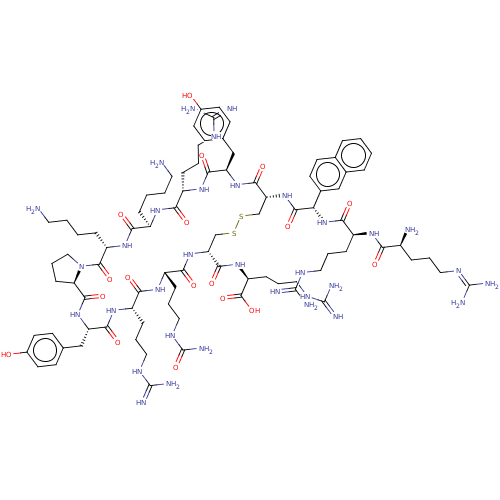 (CHEMBL2372983 | Compound T140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5048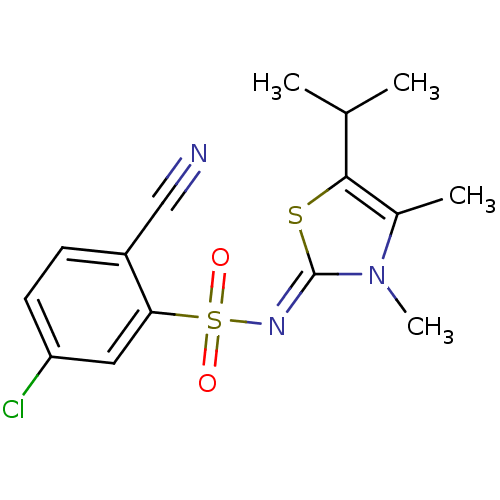 (5-Chloro-2-cyano-N-(5-isopropyl-3,4-dimethyl-1,3-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5041 (N-(5-tert-Butyl-3,4-dimethyl-1,3-thiazol-2(3H)-yli...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102037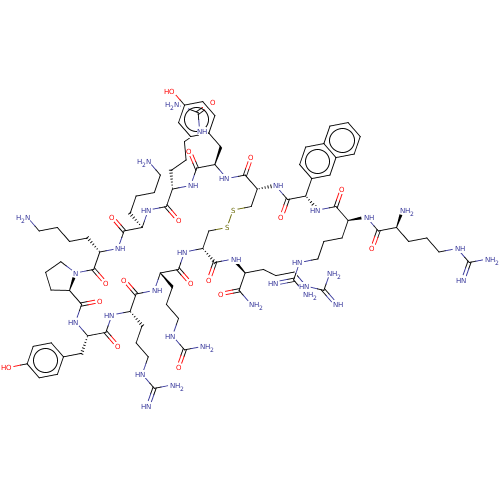 (CHEMBL2372993 | Derivative of T140 peptide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5053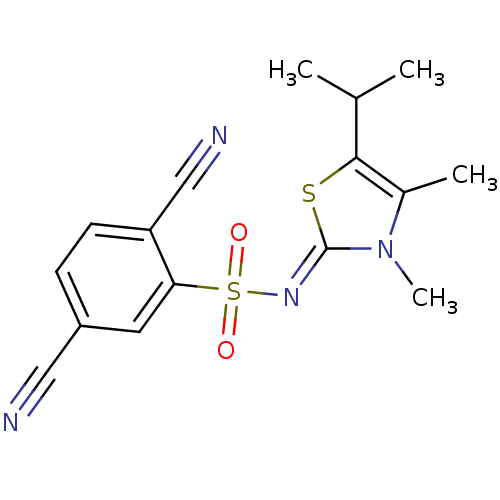 (2,5-dicyano-N-[(2Z)-3,4-dimethyl-5-(propan-2-yl)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5052 (5-bromo-N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5050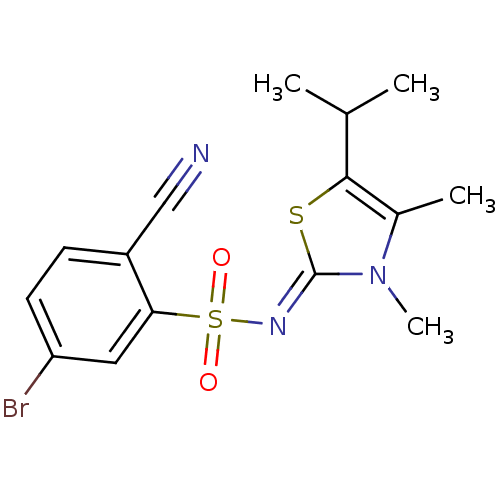 (5-Bromo-2-cyano-N-(5-isopropyl-3,4-dimethyl-1,3-th...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102036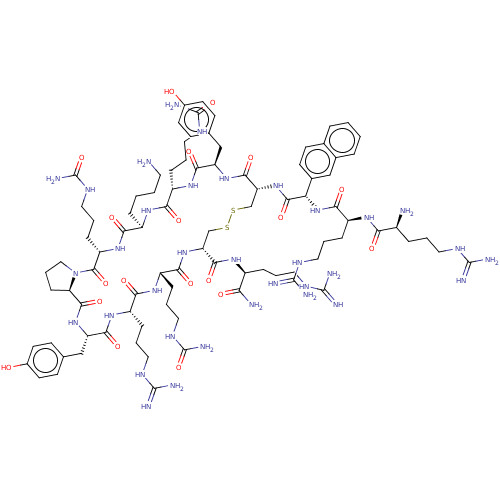 (CHEMBL2372985 | Derivative of T140 peptide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K690N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K690N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5051 (5-Chloro-N-(4-chloro-5-isopropyl-3-methyl-13-thiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM23402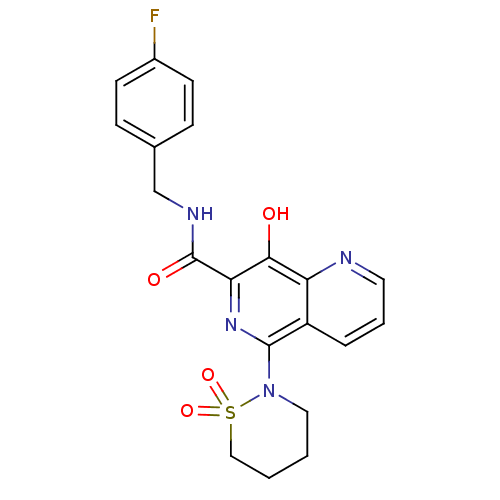 (5-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(4-fluoropheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase strand transfer activity | J Med Chem 49: 1506-8 (2006) Article DOI: 10.1021/jm0600139 BindingDB Entry DOI: 10.7270/Q2H131NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102040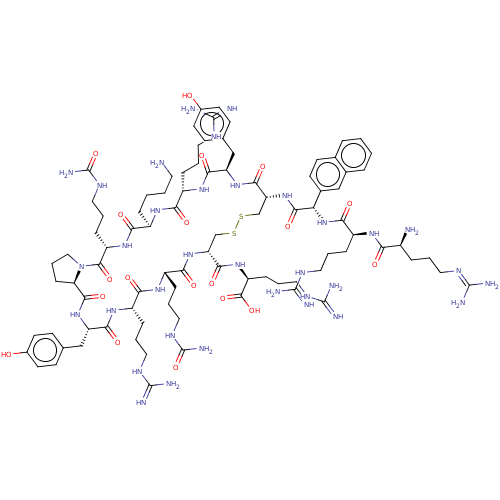 (CHEMBL2372994 | T140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102032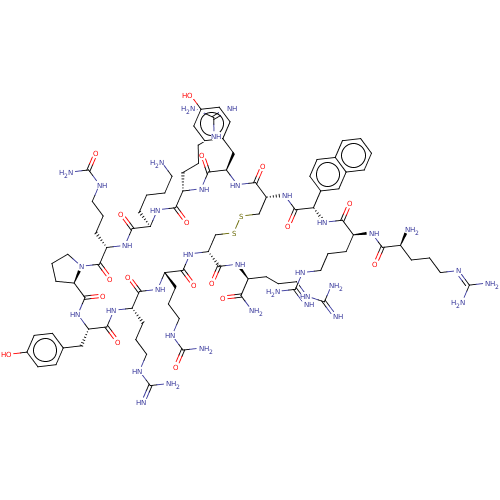 (CHEMBL2373001 | Derivative of T140 peptide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183276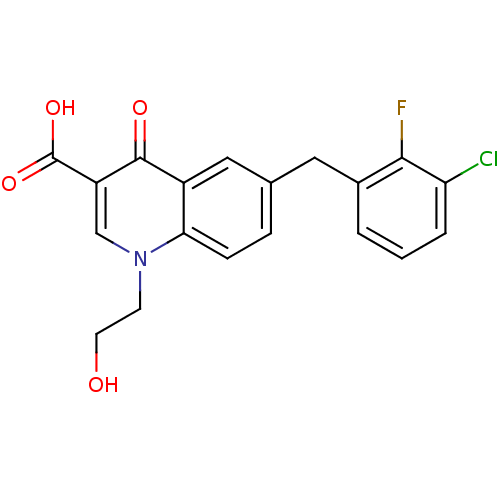 (6-(3-chloro-2-fluorobenzyl)-1-(2-hydroxyethyl)-4-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase strand transfer activity | J Med Chem 49: 1506-8 (2006) Article DOI: 10.1021/jm0600139 BindingDB Entry DOI: 10.7270/Q2H131NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5041 (N-(5-tert-Butyl-3,4-dimethyl-1,3-thiazol-2(3H)-yli...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166106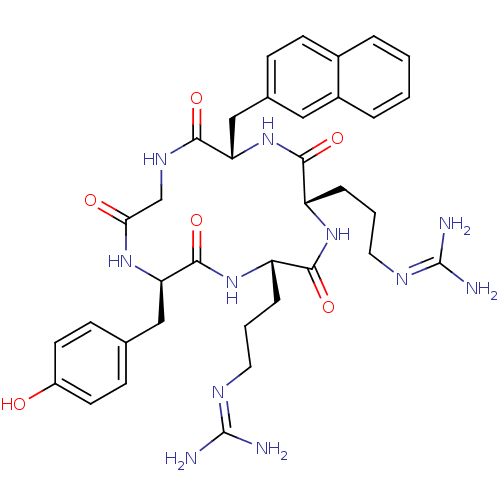 (CHEMBL436283 | N-{3-[(2S,5S,8S,14R)-5-(3-Guanidino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]SDF1 from human CXCR4 expressed in CHO cells | Bioorg Med Chem Lett 18: 4124-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.092 BindingDB Entry DOI: 10.7270/Q2PN95FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K690N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM5052 (5-bromo-N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5029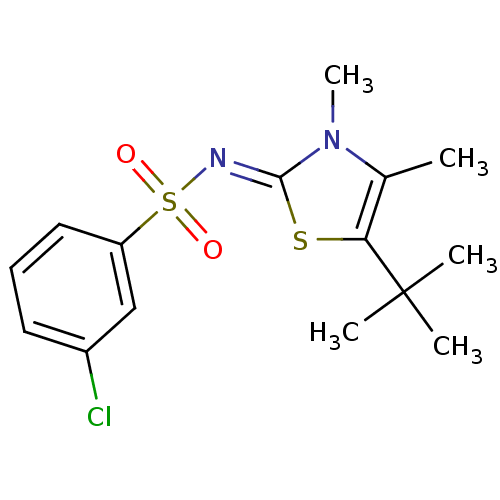 (N-(5-tert-Butyl-3,4-dimethyl-1,3-thiazol-2(3H)-yli...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 8.4 | 37 |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50235312 (6-(3-chloro-2-fluorobenzyl)-4-oxo-1,4-dihydroquino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase strand transfer activity | J Med Chem 49: 1506-8 (2006) Article DOI: 10.1021/jm0600139 BindingDB Entry DOI: 10.7270/Q2H131NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102035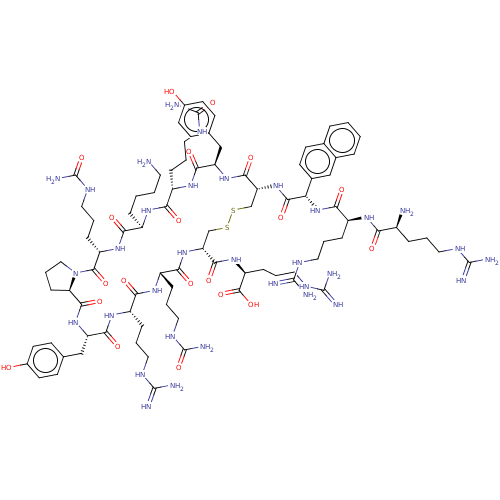 (CHEMBL2372997 | Derivative of T140 peptide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5026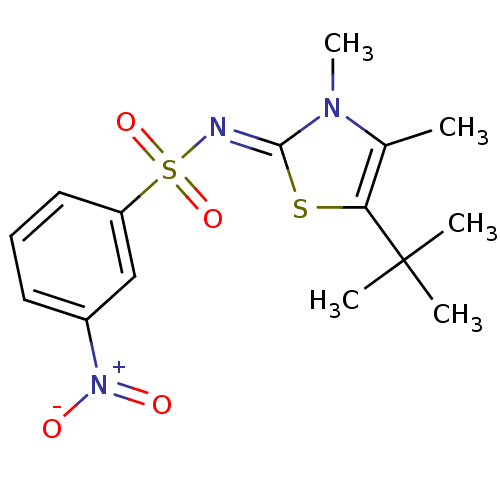 (3-substituted thiazolidene deriv. 17a | N-[(2Z)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | 8.4 | 37 |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5026 (3-substituted thiazolidene deriv. 17a | N-[(2Z)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | 8.4 | 37 |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5047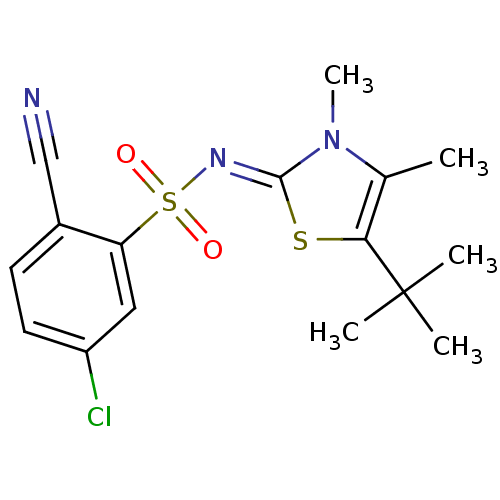 (N-(5-tert-Butyl-3,4-dimethyl-1,3-thiazol-2(3H)-yli...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5112 (N-(3,4-Dimethyl-5-isopropyl-1,3-thiazol-2(3H)-ylid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 8.4 | 37 |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5056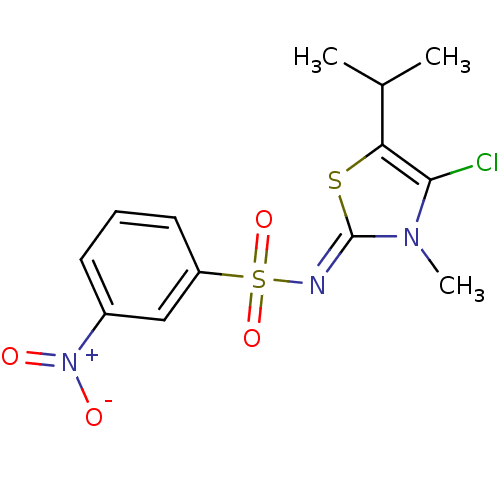 (N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5056 (N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5054 (5-Chloro-N-(4-chloro-5-isopropyl-3-methyl-1,3-thia...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5049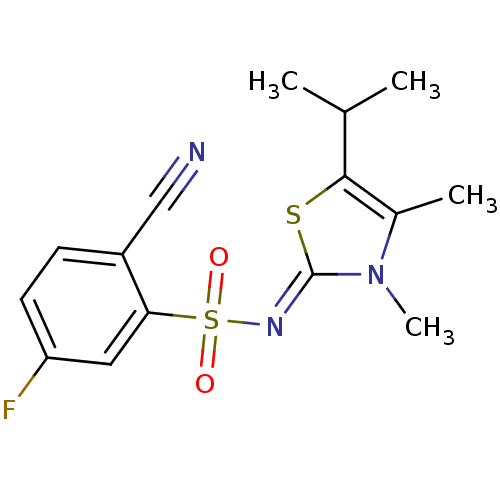 (2-cyano-N-[(2Z)-3,4-dimethyl-5-(propan-2-yl)-2,3-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5042 (2-Amino-N-(5-tert-butyl-3,4-dimethyl-1,3-thiazol-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5119 (N-(5-tert-Butyl-3-ethyl-4-methyl-1,3-thiazol-2(3H)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5056 (N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5056 (N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5120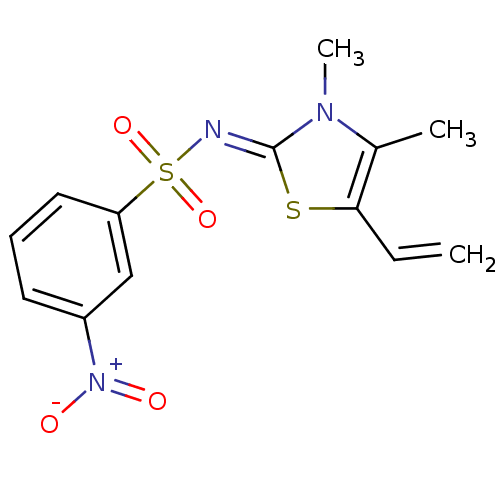 (N-(3,4-Dimethyl-5-vinyl-1,3-thiazol-2(3H)-ylidene)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K690N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM5051 (5-Chloro-N-(4-chloro-5-isopropyl-3-methyl-13-thiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5047 (N-(5-tert-Butyl-3,4-dimethyl-1,3-thiazol-2(3H)-yli...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5042 (2-Amino-N-(5-tert-butyl-3,4-dimethyl-1,3-thiazol-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 223 total ) | Next | Last >> |