 Found 508 hits with Last Name = 'kowaluk' and Initial = 'ea'
Found 508 hits with Last Name = 'kowaluk' and Initial = 'ea' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholine receptor subunit delta
(Torpedo californica) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against Nicotinic acetylcholine receptor (nAchR) |
J Med Chem 42: 1481-500 (1999)
Article DOI: 10.1021/jm9805034
BindingDB Entry DOI: 10.7270/Q2HX1DCV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50076327
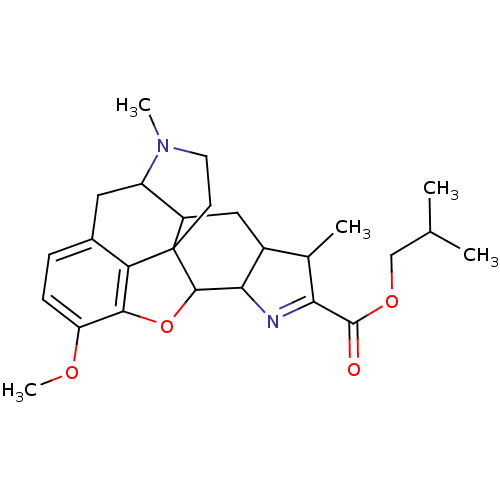
(CHEMBL34663 | isobutyl 12-methoxy-5,18-dimethyl-10...)Show SMILES COc1ccc2CC3C4CC5C(C)C(=NC5C5Oc1c2C45CCN3C)C(=O)OCC(C)C |c:13,TLB:18:19:8:23.21.22,24:23:8:19.5.6| Show InChI InChI=1S/C26H34N2O4/c1-13(2)12-31-25(29)21-14(3)16-11-17-18-10-15-6-7-19(30-5)23-20(15)26(17,8-9-28(18)4)24(32-23)22(16)27-21/h6-7,13-14,16-18,22,24H,8-12H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Opioid receptor delta 1 agonist activity was determined |
J Med Chem 42: 1481-500 (1999)
Article DOI: 10.1021/jm9805034
BindingDB Entry DOI: 10.7270/Q2HX1DCV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2C
(Rattus norvegicus (Rat)) | BDBM50062599
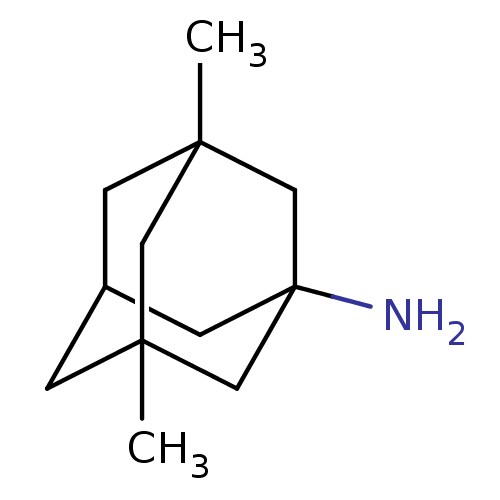
(3,5-Dimethyl-adamantan-1-ylamine | CHEMBL807 | EN3...)Show SMILES CC12CC3CC(C)(C1)CC(N)(C3)C2 |TLB:7:1:4.5.8:11,10:9:4:2.7.1,0:1:4:8.9.11,THB:7:5:11:2.1.12,12:1:4:8.9.11,12:9:4:2.7.1,6:5:11:2.1.12| Show InChI InChI=1S/C12H21N/c1-10-3-9-4-11(2,6-10)8-12(13,5-9)7-10/h9H,3-8,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound with the N-methyl-D-aspartate glutamate receptor blocking activity |
J Med Chem 42: 1481-500 (1999)
Article DOI: 10.1021/jm9805034
BindingDB Entry DOI: 10.7270/Q2HX1DCV |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50176259
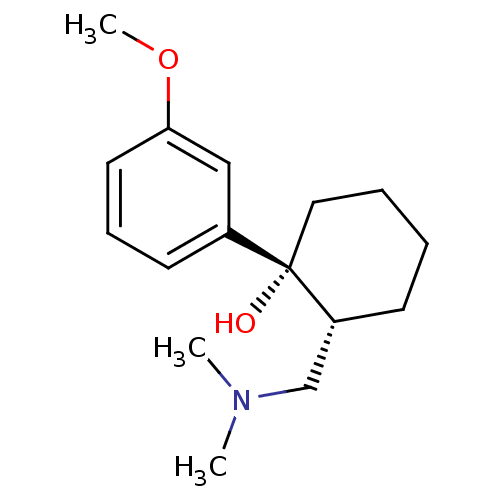
((1R,2R)-2-[(dimethylamino)methyl]-1-(3-methoxyphen...)Show InChI InChI=1S/C16H25NO2/c1-17(2)12-14-7-4-5-10-16(14,18)13-8-6-9-15(11-13)19-3/h6,8-9,11,14,18H,4-5,7,10,12H2,1-3H3/t14-,16+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Opioid receptor mu 1 agonist activity with monoamine (NE, 5-HT) uptake-blocking activity in the 0.8-1 uM range |
J Med Chem 42: 1481-500 (1999)
Article DOI: 10.1021/jm9805034
BindingDB Entry DOI: 10.7270/Q2HX1DCV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
VIP peptides
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 8
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Chloride channel protein 1
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Sodium/potassium-transporting ATPase subunit alpha-1
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-1 subunit
(Homo sapiens (Human)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Calcium release-activated calcium channel protein 1
(Homo sapiens (Human)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(RAT) | BDBM50094703

(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 1156-64 (2000)
BindingDB Entry DOI: 10.7270/Q2DF6PSP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data