

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50280415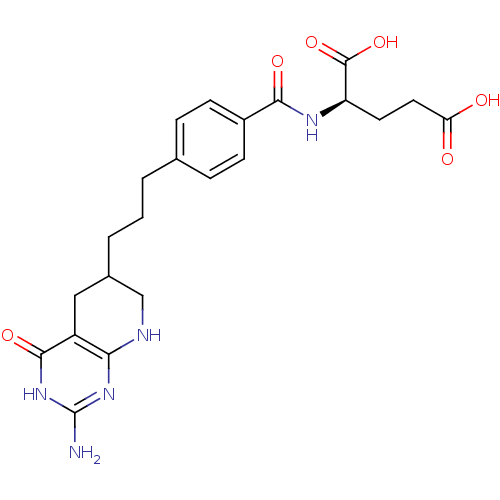 ((R)-2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.0000190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM22590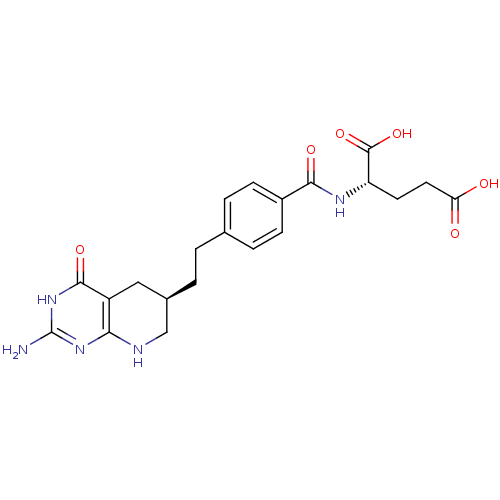 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 0.000120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50280414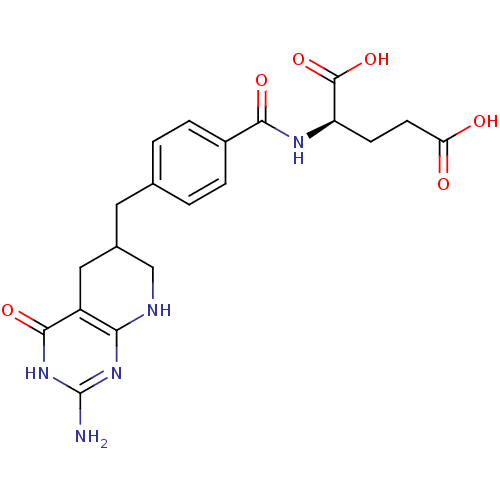 ((R)-2-[4-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50003896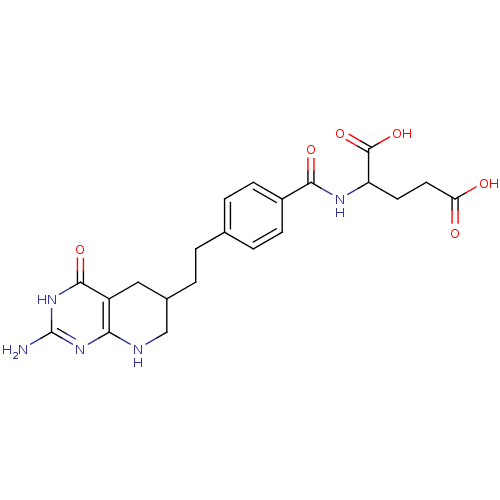 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthase | J Med Chem 35: 4450-4 (1992) BindingDB Entry DOI: 10.7270/Q2RR1ZV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM22590 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibition of trifunctional glycinamide ribonucleotide formyltransferase (GARFT) isolated from murine L1210 leukemia cells | Bioorg Med Chem Lett 7: 453-456 (1997) Article DOI: 10.1016/S0960-894X(97)00041-3 BindingDB Entry DOI: 10.7270/Q2HT2PB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibition of trifunctional glycinamide ribonucleotide formyltransferase (GARFT) isolated from murine L1210 leukemia cells | Bioorg Med Chem Lett 7: 453-456 (1997) Article DOI: 10.1016/S0960-894X(97)00041-3 BindingDB Entry DOI: 10.7270/Q2HT2PB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50291133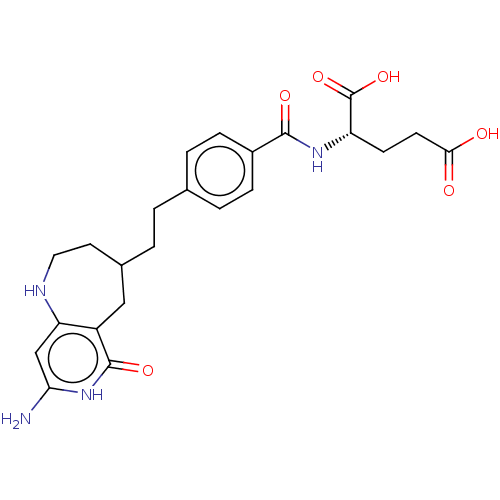 (2-{(S)-4-[2-(8-Amino-6-oxo-2,3,4,5,6,7-hexahydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibition of trifunctional glycinamide ribonucleotide formyltransferase (GARFT) isolated from murine L1210 leukemia cells | Bioorg Med Chem Lett 7: 453-456 (1997) Article DOI: 10.1016/S0960-894X(97)00041-3 BindingDB Entry DOI: 10.7270/Q2HT2PB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18796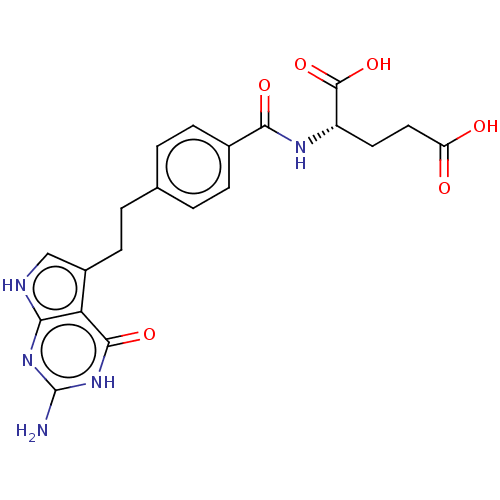 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthase | J Med Chem 35: 4450-4 (1992) BindingDB Entry DOI: 10.7270/Q2RR1ZV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Bos taurus (Cattle)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against beef liver dihydrofolate reductase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50026383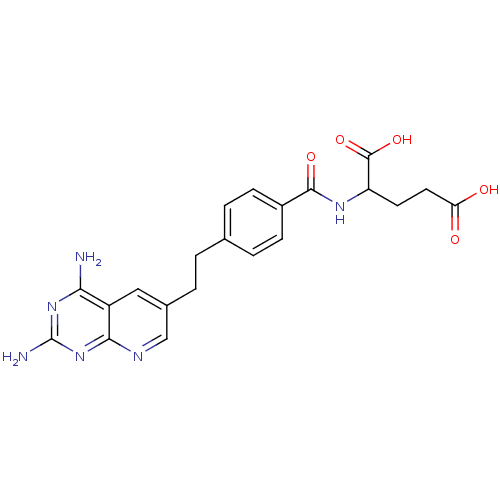 (2-{4-[2-(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against beef liver dihydrofolate reductase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50026384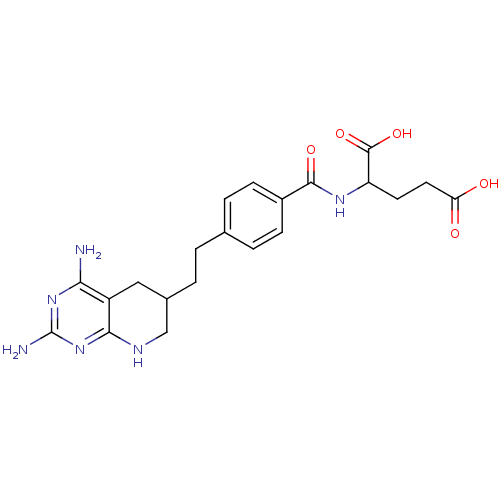 (2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido[2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against beef liver dihydrofolate reductase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50367251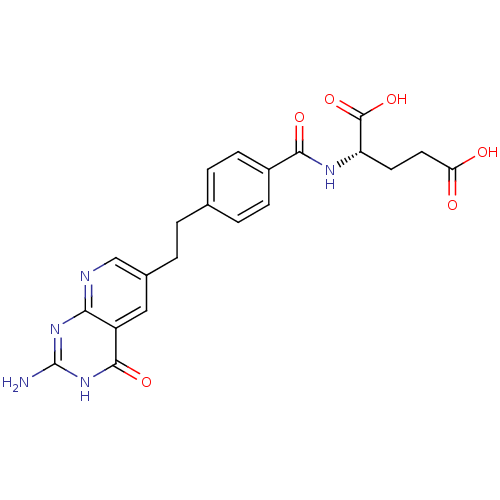 (CHEMBL350097) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against beef liver dihydrofolate reductase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50367251 (CHEMBL350097) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Lactobacillus casei thymidylate synthetase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50026383 (2-{4-[2-(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Lactobacillus casei thymidylate synthetase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Lactobacillus casei thymidylate synthetase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against beef liver dihydrofolate reductase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50026384 (2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido[2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Lactobacillus casei thymidylate synthetase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Lactobacillus casei thymidylate synthetase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||