 Found 457 hits with Last Name = 'burstein' and Initial = 'es'
Found 457 hits with Last Name = 'burstein' and Initial = 'es' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570555
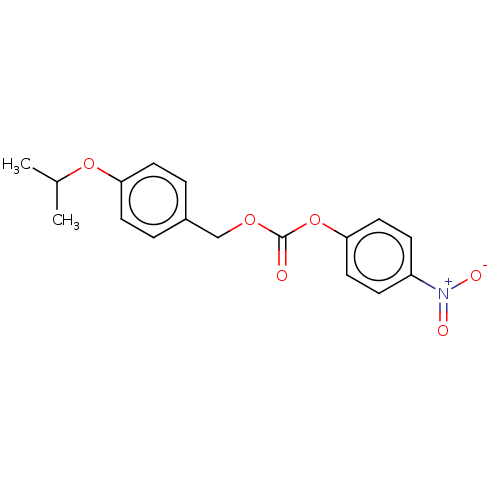
(US11440884, Example 18 | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)Oc2ccc(cc2)[N+]([O-])=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86187
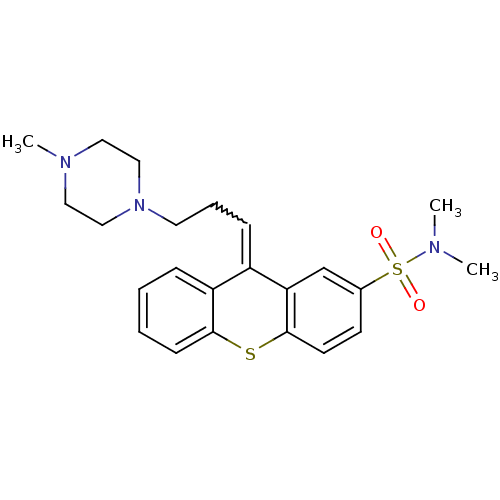
(CAS_22189-31-7 | NSC_5454 | THIOTHIXENE)Show SMILES CN(C)S(=O)(=O)c1ccc2Sc3ccccc3C(=CCCN3CCN(C)CC3)c2c1 |w:18.19| Show InChI InChI=1S/C23H29N3O2S2/c1-24(2)30(27,28)18-10-11-23-21(17-18)19(20-7-4-5-9-22(20)29-23)8-6-12-26-15-13-25(3)14-16-26/h4-5,7-11,17H,6,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50232153
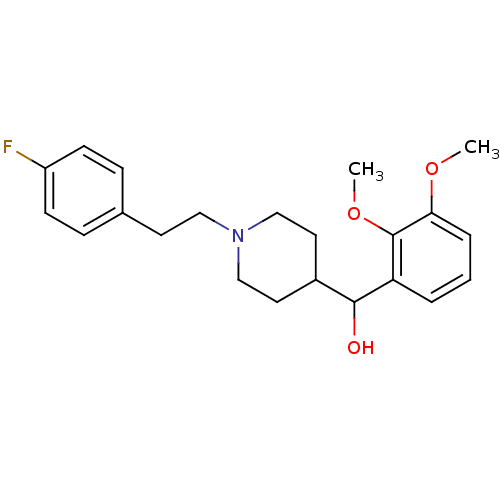
((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...)Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | -55.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570569
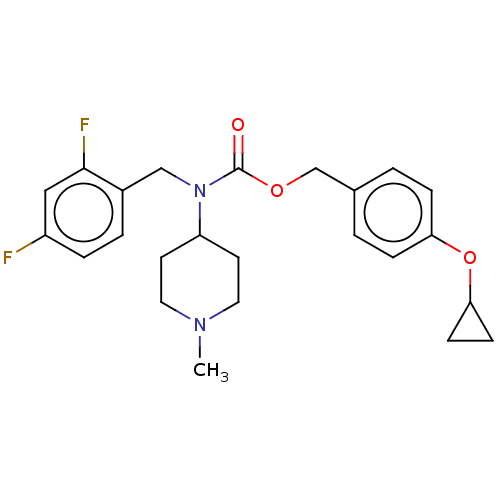
(US11440884, Example 30)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OC2CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM139371
(eplivanserin)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]-[#8]-[#7]\[#6](=[#6]\[#6]=[#6]-1\[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)-c1ccccc1F |c:11,15| Show InChI InChI=1S/C19H21FN2O2/c1-22(2)13-14-24-21-19(17-5-3-4-6-18(17)20)12-9-15-7-10-16(23)11-8-15/h3-12,21H,13-14H2,1-2H3/b19-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | -54.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570568
(US11440884, Example 29)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OCC(C)(C)O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570567
(US11440884, Example 28)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OC(CF)CF)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0830 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570571
(US11440884, Example 32)Show SMILES COc1cc(F)c(F)cc1CN(C1CCN(C)CC1)C(=O)Cc1ccc(OC(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570574
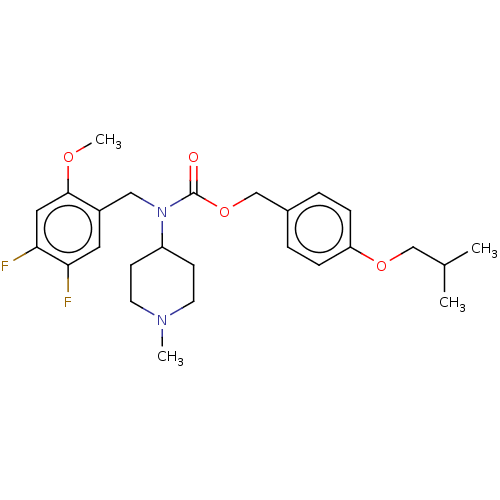
(US11440884, Example 35)Show SMILES COc1cc(F)c(F)cc1CN(C1CCN(C)CC1)C(=O)OCc1ccc(OCC(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570602
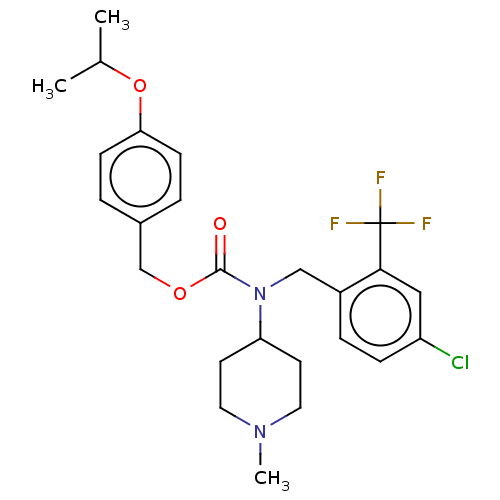
(US11440884, Example 54)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(Cl)cc2C(F)(F)F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570603
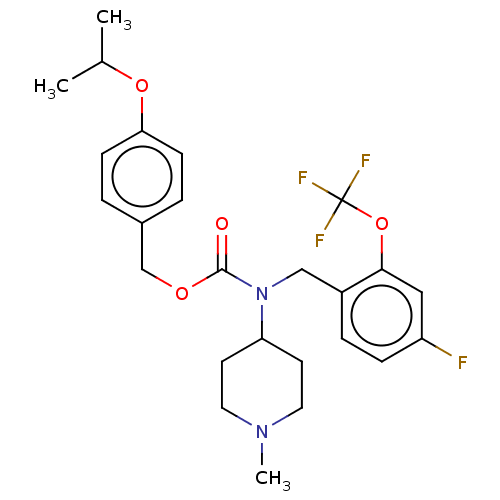
(US11440884, Example 55)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2OC(F)(F)F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570616
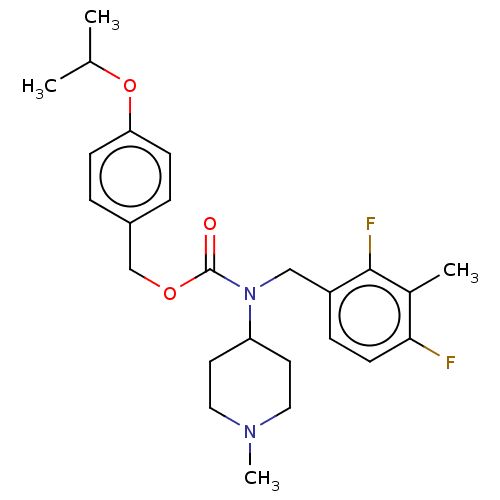
(US11440884, Example 67)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)c(C)c2F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570619
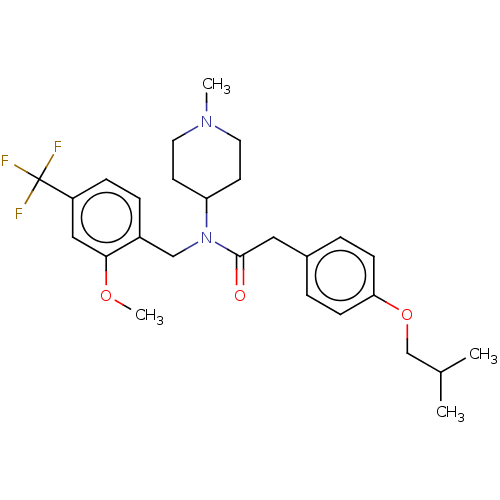
(US11440884, Example 70)Show SMILES COc1cc(ccc1CN(C1CCN(C)CC1)C(=O)Cc1ccc(OCC(C)C)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570621
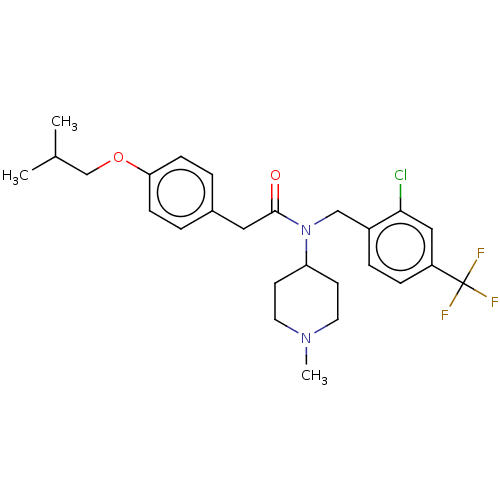
(US11440884, Example 71)Show SMILES CC(C)COc1ccc(CC(=O)N(Cc2ccc(cc2Cl)C(F)(F)F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570622
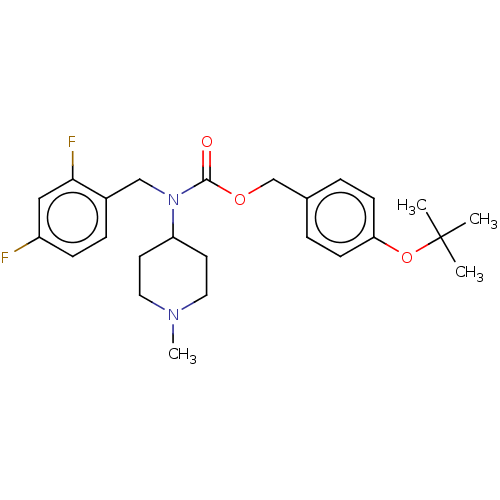
(US11440884, Example 72)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OC(C)(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570627
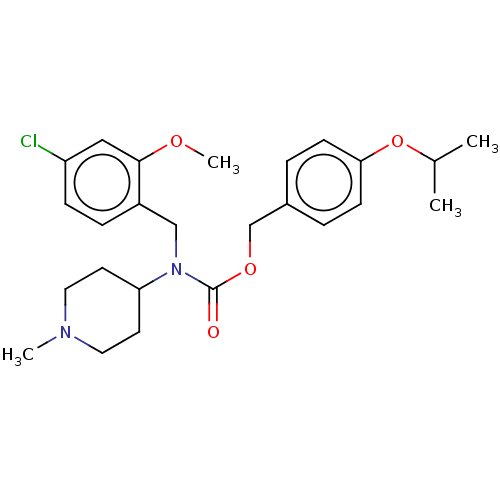
(US11440884, Example 76)Show SMILES COc1cc(Cl)ccc1CN(C1CCN(C)CC1)C(=O)OCc1ccc(OC(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570628

(US11440884, Example 77)Show SMILES COc1cc(ccc1CN(C1CCN(C)CC1)C(=O)OCc1ccc(OC(C)C)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570517
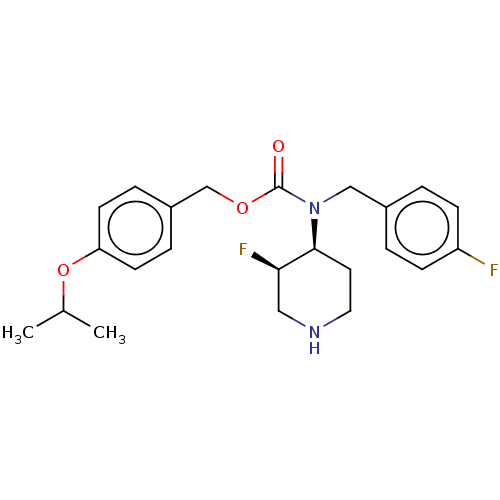
(US11440884, Example 3a | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@H]2CCNC[C@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570524
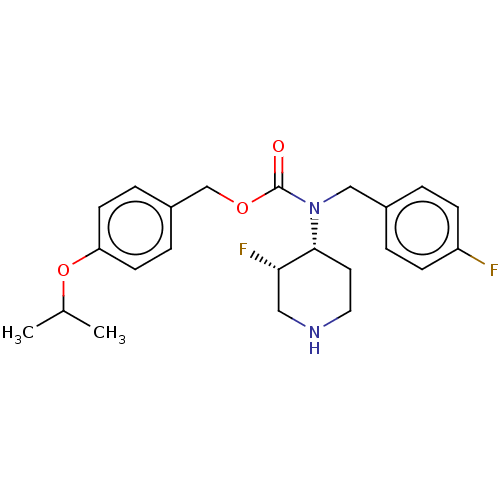
(US11440884, Example 3b | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@@H]2CCNC[C@@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570525
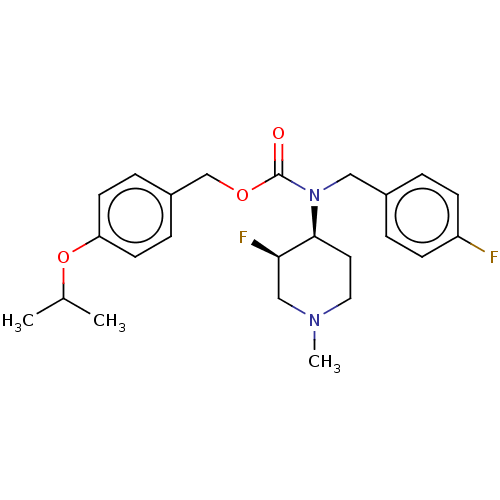
( [4-(propan-2-yloxy)phenyl]methyl N-[(3R,4S)-3-flu...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@H]2CCN(C)C[C@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570526
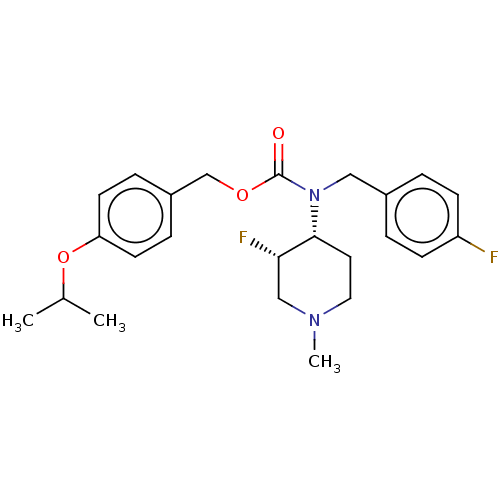
(US11440884, Example 4b | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@@H]2CCN(C)C[C@@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM84734
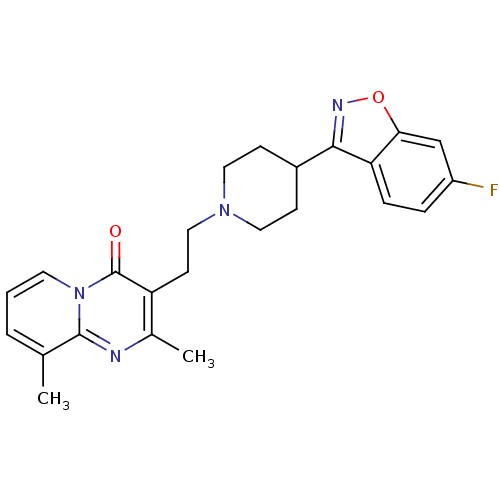
(CAS_129029-23-8 | NSC_71351 | Ocaperidone)Show SMILES Cc1cccn2c1nc(C)c(CCN1CCC(CC1)c1noc3cc(F)ccc13)c2=O Show InChI InChI=1S/C24H25FN4O2/c1-15-4-3-10-29-23(15)26-16(2)19(24(29)30)9-13-28-11-7-17(8-12-28)22-20-6-5-18(25)14-21(20)31-27-22/h3-6,10,14,17H,7-9,11-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50252513
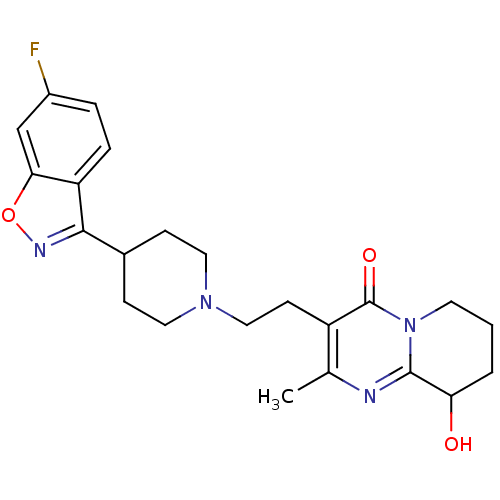
(3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1...)Show SMILES Cc1nc2C(O)CCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM81486
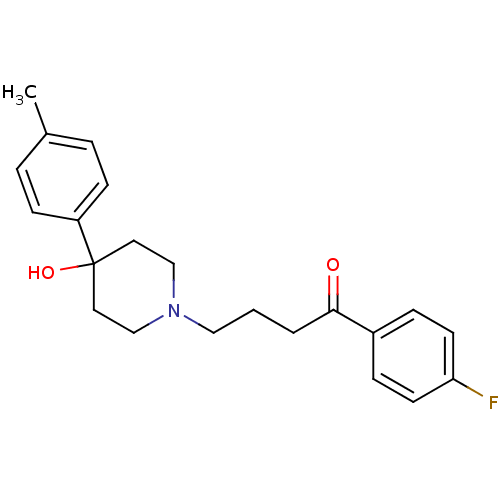
(CAS_1050-79-9 | MOPERONE | NSC_4249)Show SMILES Cc1ccc(cc1)C1(O)CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H26FNO2/c1-17-4-8-19(9-5-17)22(26)12-15-24(16-13-22)14-2-3-21(25)18-6-10-20(23)11-7-18/h4-11,26H,2-3,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570545
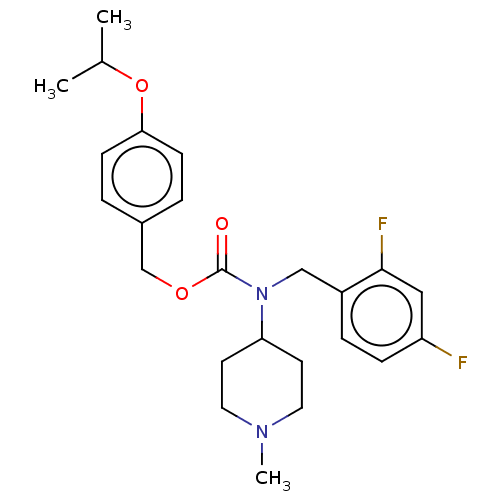
(US11440884, Example 13)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570516
(US11440884, Example 2)Show SMILES CC(C)COc1ccc(COC(=O)N(Cc2ccc(F)cc2)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570587

(US11440884, Example 39)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OCCF)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570566
(US11440884, Example 27)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OCCCF)cc1 |$;;;;;;;;;;;;;;;;;;;;;;;;;;;;;F;;$| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570565
(US11440884, Example 25)Show SMILES CCOc1ccc(COC(=O)N(Cc2ccc(F)cc2F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29098
(dibenzothiazepine, 12e)Show SMILES CCCCNC(=O)c1ccc2Sc3ccccc3C(=Nc2c1)c1ccc(Cl)cc1 |c:19| Show InChI InChI=1S/C24H21ClN2OS/c1-2-3-14-26-24(28)17-10-13-22-20(15-17)27-23(16-8-11-18(25)12-9-16)19-6-4-5-7-21(19)29-22/h4-13,15H,2-3,14H2,1H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29100

(dibenzothiazepine, 12h)Show SMILES CCCCNC(=O)c1ccc2Sc3ccccc3C(=Nc2c1)c1ccc(F)c(Cl)c1 |c:19| Show InChI InChI=1S/C24H20ClFN2OS/c1-2-3-12-27-24(29)16-9-11-22-20(14-16)28-23(15-8-10-19(26)18(25)13-15)17-6-4-5-7-21(17)30-22/h4-11,13-14H,2-3,12H2,1H3,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB
| Assay Description
IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... |
J Med Chem 52: 1975-82 (2009)
Article DOI: 10.1021/jm801534c
BindingDB Entry DOI: 10.7270/Q25Q4TFP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86722
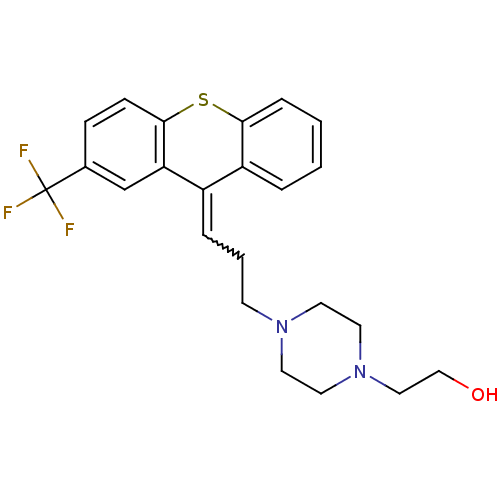
(CAS_53772-82-0 | CAS_53772-85-3 | FLUPENTHIXOL, Al...)Show SMILES OCCN1CCN(CCC=C2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 |w:9.8| Show InChI InChI=1S/C23H25F3N2OS/c24-23(25,26)17-7-8-22-20(16-17)18(19-4-1-2-6-21(19)30-22)5-3-9-27-10-12-28(13-11-27)14-15-29/h1-2,4-8,16,29H,3,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM78433
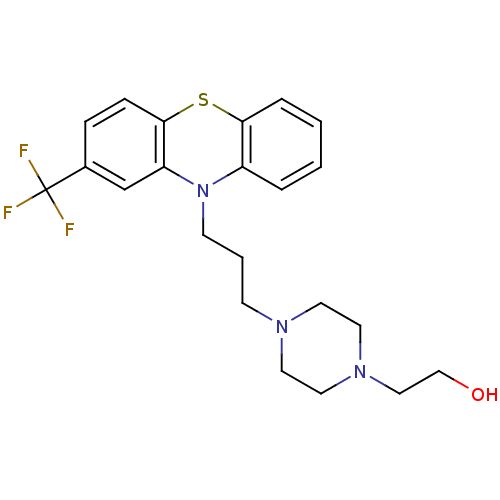
(2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...)Show SMILES OCCN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C22H26F3N3OS/c23-22(24,25)17-6-7-21-19(16-17)28(18-4-1-2-5-20(18)30-21)9-3-8-26-10-12-27(13-11-26)14-15-29/h1-2,4-7,16,29H,3,8-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM26948
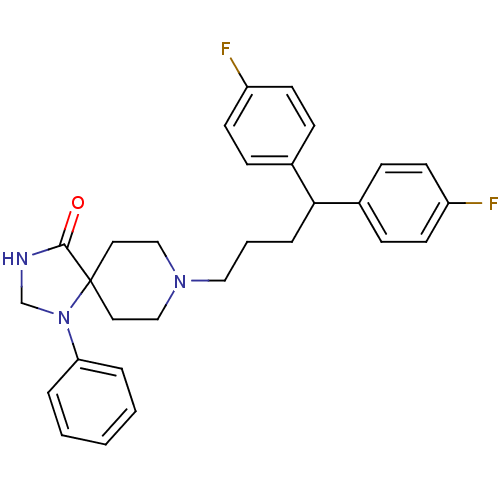
(8-[4,4-bis(4-fluorophenyl)butyl]-1-phenyl-1,3,8-tr...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C29H31F2N3O/c30-24-12-8-22(9-13-24)27(23-10-14-25(31)15-11-23)7-4-18-33-19-16-29(17-20-33)28(35)32-21-34(29)26-5-2-1-3-6-26/h1-3,5-6,8-15,27H,4,7,16-21H2,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM26948

(8-[4,4-bis(4-fluorophenyl)butyl]-1-phenyl-1,3,8-tr...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C29H31F2N3O/c30-24-12-8-22(9-13-24)27(23-10-14-25(31)15-11-23)7-4-18-33-19-16-29(17-20-33)28(35)32-21-34(29)26-5-2-1-3-6-26/h1-3,5-6,8-15,27H,4,7,16-21H2,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM22872
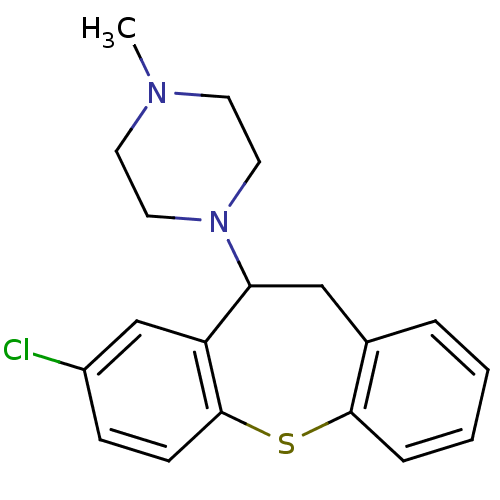
(1-(3-chloro-5,6-dihydrobenzo[b][1]benzothiepin-5-y...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM78434
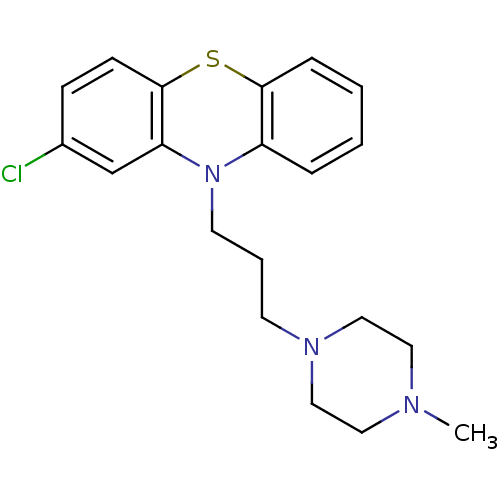
(2-chloranyl-10-[3-(4-methylpiperazin-1-yl)propyl]p...)Show InChI InChI=1S/C20H24ClN3S/c1-22-11-13-23(14-12-22)9-4-10-24-17-5-2-3-6-19(17)25-20-8-7-16(21)15-18(20)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86723
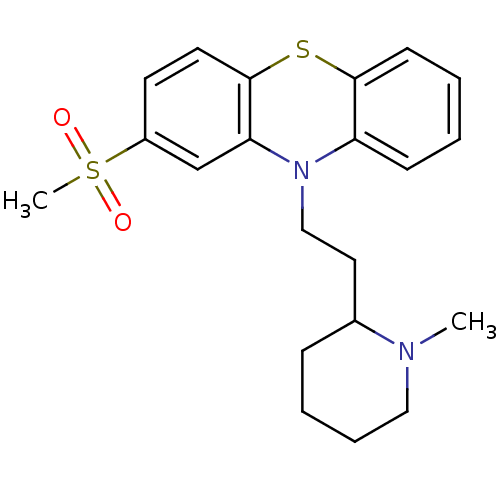
(CAS_14759-06-9 | NSC_31765 | sulforidazine)Show SMILES CN1CCCCC1CCN1c2ccccc2Sc2ccc(cc12)S(C)(=O)=O Show InChI InChI=1S/C21H26N2O2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)26-21-11-10-17(15-19(21)23)27(2,24)25/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86187

(CAS_22189-31-7 | NSC_5454 | THIOTHIXENE)Show SMILES CN(C)S(=O)(=O)c1ccc2Sc3ccccc3C(=CCCN3CCN(C)CC3)c2c1 |w:18.19| Show InChI InChI=1S/C23H29N3O2S2/c1-24(2)30(27,28)18-10-11-23-21(17-18)19(20-7-4-5-9-22(20)29-23)8-6-12-26-15-13-25(3)14-16-26/h4-5,7-11,17H,6,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM81493
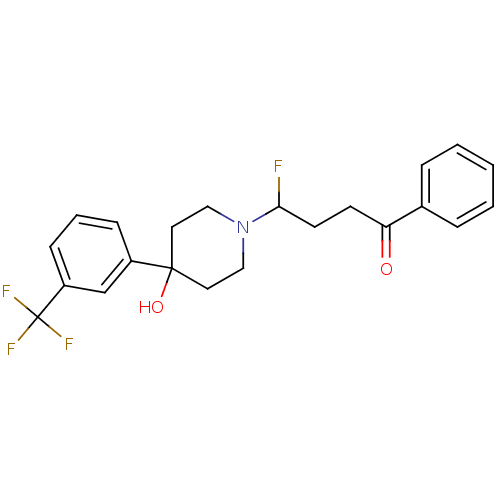
(CAS_749-13-3 | TRIFLUORPERIDOL)Show SMILES OC1(CCN(CC1)C(F)CCC(=O)c1ccccc1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H23F4NO2/c23-20(10-9-19(28)16-5-2-1-3-6-16)27-13-11-21(29,12-14-27)17-7-4-8-18(15-17)22(24,25)26/h1-8,15,20,29H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM81484
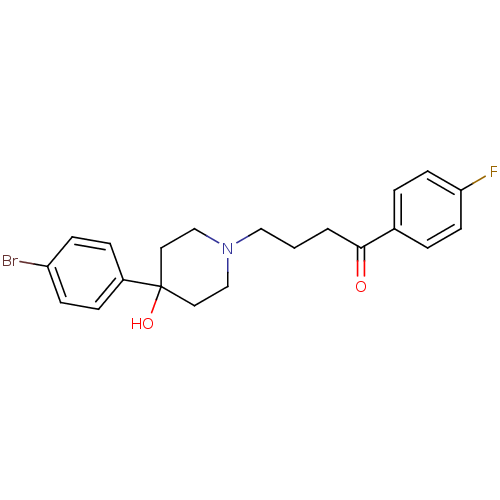
(BROMPERIDOL | Bromoperidol | CAS_2448 | NSC_2448)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Br)cc1 Show InChI InChI=1S/C21H23BrFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM22872

(1-(3-chloro-5,6-dihydrobenzo[b][1]benzothiepin-5-y...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50334150
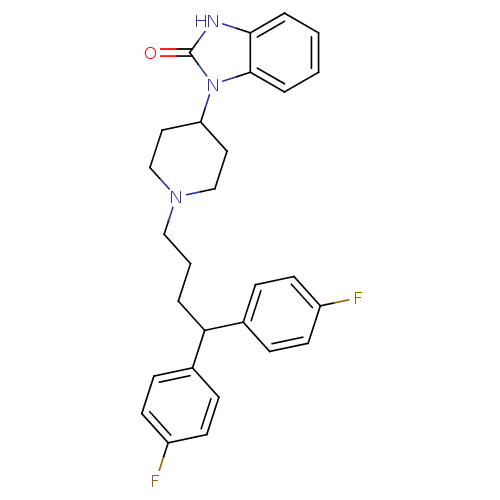
(1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570515
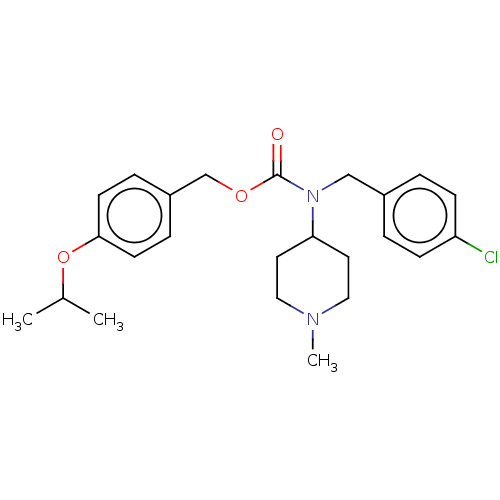
(US11440884, Example 1)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(Cl)cc2)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570527
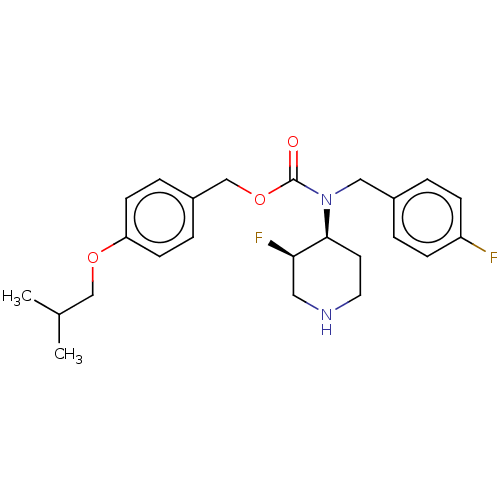
(US11440884, Example 5a | [4-(2-methylpropoxy)pheny...)Show SMILES CC(C)COc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@H]2CCNC[C@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570528
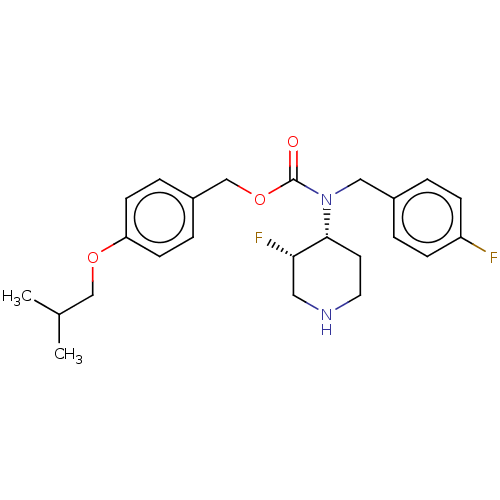
(US11440884, Example 5b | [4-(2-methylpropoxy)pheny...)Show SMILES CC(C)COc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@@H]2CCNC[C@@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data