

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052440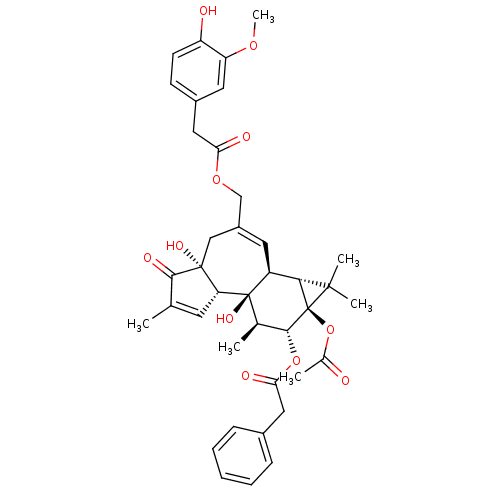 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory constant for RTX binding to rat spinal cord | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50052440 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory constant for RTX binding to porcine spinal cord | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50052440 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory constant for RTX binding to human spinal cord | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.450 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bern , Bühlstrasse 28, CH-3012 Bern, Switzerland. | Assay Description In this assay, 18 ug of crude membrane expressing hCB1 or hCB2 receptors were re-suspended in 300 uL of binding buffer containing 50 mM Tris-HCl, 2.5... | ACS Chem Biol 9: 1499-507 (2014) Article DOI: 10.1021/cb500177c BindingDB Entry DOI: 10.7270/Q2T15295 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` del Piemonte Orientale Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human recombinant CB2 receptor expressed in HEK293 cells membrane incubated for 90 mins | J Nat Prod 74: 2019-22 (2011) Article DOI: 10.1021/np200500p BindingDB Entry DOI: 10.7270/Q2BR8SKG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.5 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bern , Bühlstrasse 28, CH-3012 Bern, Switzerland. | Assay Description In this assay, 18 ug of crude membrane expressing hCB1 or hCB2 receptors were re-suspended in 300 uL of binding buffer containing 50 mM Tris-HCl, 2.5... | ACS Chem Biol 9: 1499-507 (2014) Article DOI: 10.1021/cb500177c BindingDB Entry DOI: 10.7270/Q2T15295 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` del Piemonte Orientale Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human recombinant CB1 receptor expressed in HEK293 cells membrane incubated for 90 mins | J Nat Prod 74: 2019-22 (2011) Article DOI: 10.1021/np200500p BindingDB Entry DOI: 10.7270/Q2BR8SKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50196337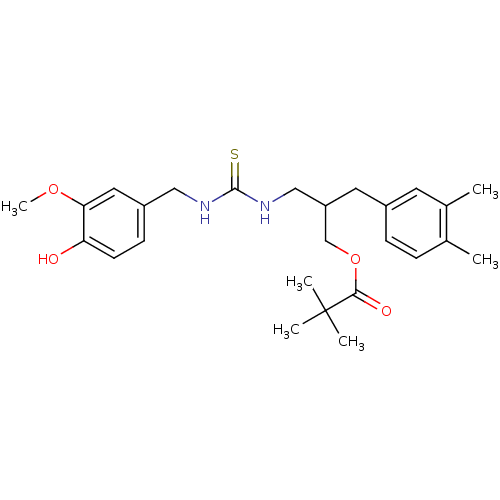 (2,2-Dimethyl-propionic acid 2-(3,4-dimethyl-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hospital of the University of Pennsylvania Curated by ChEMBL | Assay Description Agonistic activity against vanilloid receptor 1 (TRPV1) | J Med Chem 47: 2717-23 (2004) Article DOI: 10.1021/jm030560j BindingDB Entry DOI: 10.7270/Q2TQ6498 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29079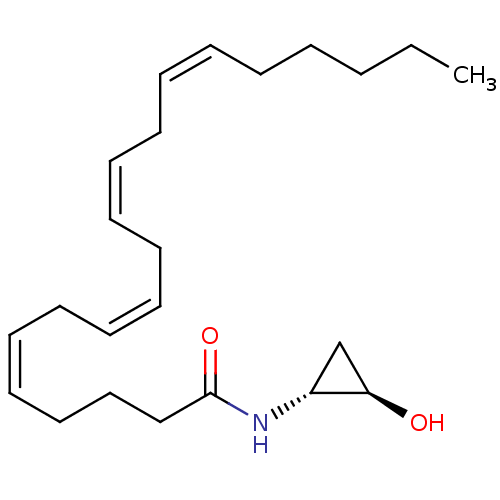 (cyclopropanolamide, 12a | cyclopropanolamide, rac-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180036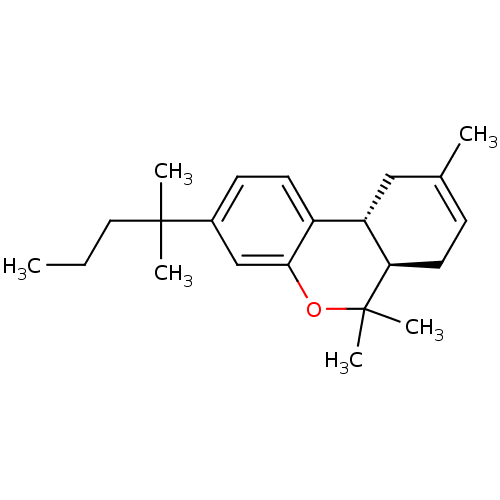 ((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bern , Bühlstrasse 28, CH-3012 Bern, Switzerland. | Assay Description In this assay, 18 ug of crude membrane expressing hCB1 or hCB2 receptors were re-suspended in 300 uL of binding buffer containing 50 mM Tris-HCl, 2.5... | ACS Chem Biol 9: 1499-507 (2014) Article DOI: 10.1021/cb500177c BindingDB Entry DOI: 10.7270/Q2T15295 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29078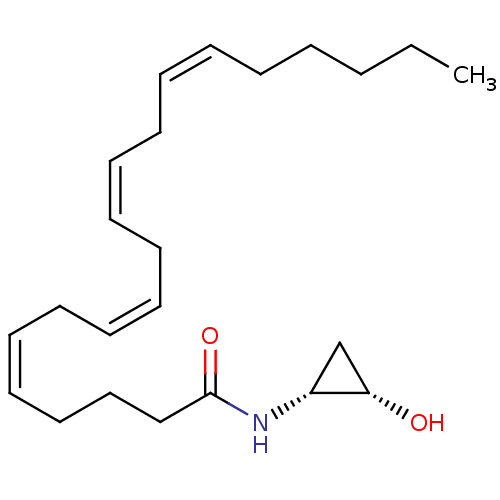 (cyclopropanolamide, 13a | cyclopropanolamide, rac-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29087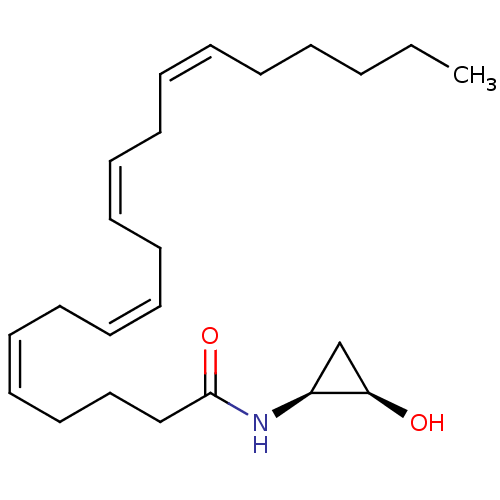 (cyclopropanolamide, 14a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB1 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00513 BindingDB Entry DOI: 10.7270/Q2251P0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50377913 (HYPERFORIN) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano Curated by ChEMBL | Assay Description Displacement of [3H]SR12813 from human PXR by scintillation proximity competition binding assay | J Nat Prod 65: 433-8 (2002) BindingDB Entry DOI: 10.7270/Q29P32H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29078 (cyclopropanolamide, 13a | cyclopropanolamide, rac-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 27 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29088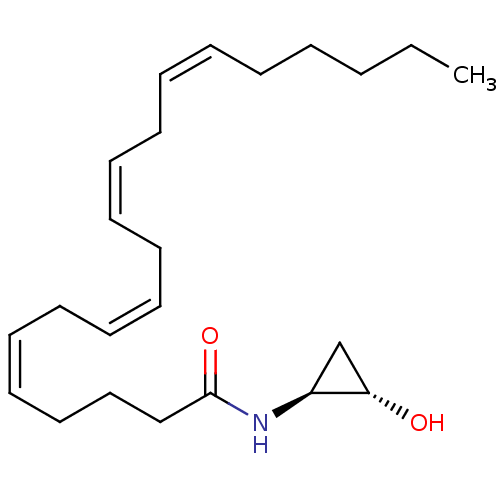 (cyclopropanolamide, 11a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29079 (cyclopropanolamide, 12a | cyclopropanolamide, rac-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 32 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00513 BindingDB Entry DOI: 10.7270/Q2251P0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Farmaceutiche Curated by ChEMBL | Assay Description Binding affinity to human recombinant CB1 receptor expressed in COS cells | J Med Chem 49: 2333-8 (2006) Article DOI: 10.1021/jm051240y BindingDB Entry DOI: 10.7270/Q2862G15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50185034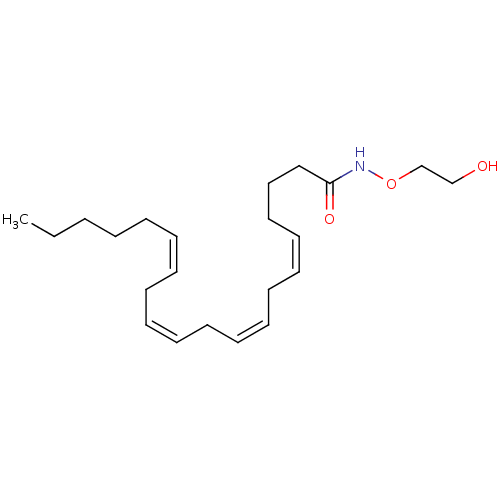 (CHEMBL382444 | N-arachidonoyl-O-(2-hydroxyethyl)hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Farmaceutiche Curated by ChEMBL | Assay Description Binding affinity to human recombinant CB2 receptor expressed in COS cells | J Med Chem 49: 2333-8 (2006) Article DOI: 10.1021/jm051240y BindingDB Entry DOI: 10.7270/Q2862G15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50577909 (CHEMBL4845760) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00513 BindingDB Entry DOI: 10.7270/Q2251P0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29078 (cyclopropanolamide, 13a | cyclopropanolamide, rac-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 168 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM22988 ((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Farmaceutiche Curated by ChEMBL | Assay Description Binding affinity to human recombinant CB2 receptor expressed in COS cells | J Med Chem 49: 2333-8 (2006) Article DOI: 10.1021/jm051240y BindingDB Entry DOI: 10.7270/Q2862G15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50353094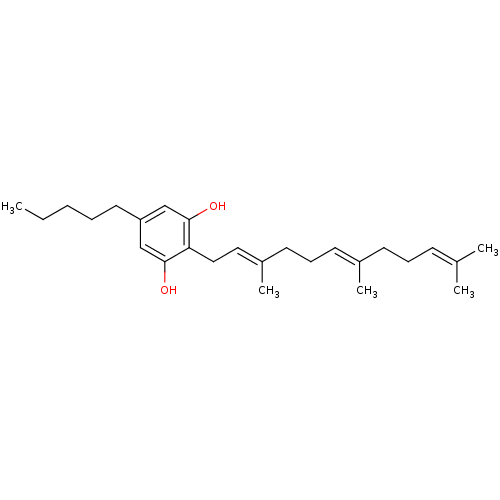 (CHEMBL1823117) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` del Piemonte Orientale Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human recombinant CB2 receptor expressed in HEK293 cells membrane incubated for 90 mins | J Nat Prod 74: 2019-22 (2011) Article DOI: 10.1021/np200500p BindingDB Entry DOI: 10.7270/Q2BR8SKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29079 (cyclopropanolamide, 12a | cyclopropanolamide, rac-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29087 (cyclopropanolamide, 14a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 190 | -39.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052437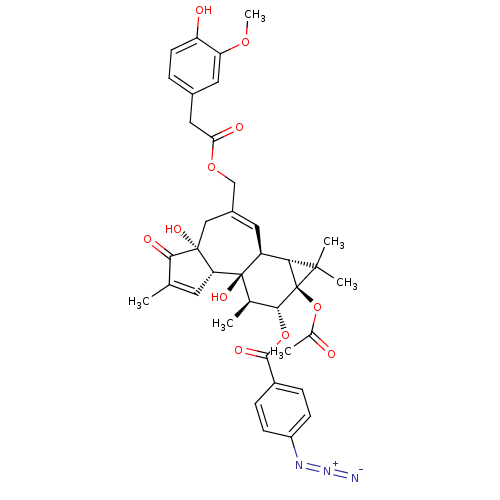 (4-Azido-benzoic acid (1aR,1bS,4aR,7aS,7bS,8R,9R,9a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052439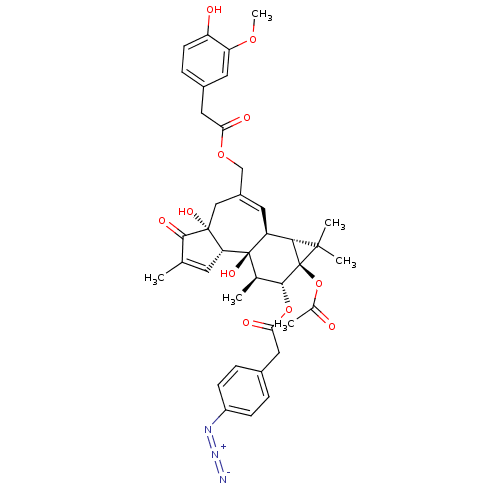 ((4-Azido-phenyl)-acetic acid (1aR,1bS,4aR,7aS,7bS,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29078 (cyclopropanolamide, 13a | cyclopropanolamide, rac-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50577909 (CHEMBL4845760) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB1 receptor transfected in CHO cells measured for 1.5 hrs by liquid scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00513 BindingDB Entry DOI: 10.7270/Q2251P0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29079 (cyclopropanolamide, 12a | cyclopropanolamide, rac-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 240 | -38.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29088 (cyclopropanolamide, 11a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052443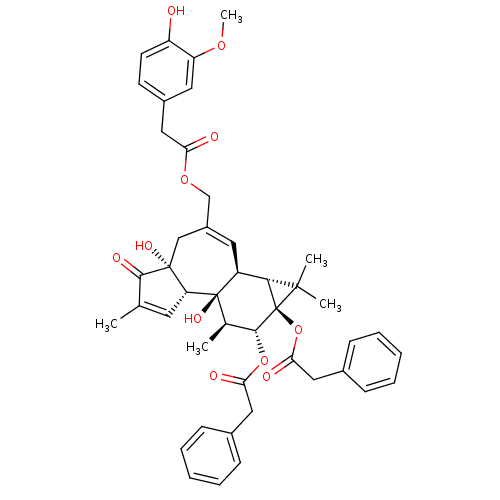 (CHEMBL320485 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM86536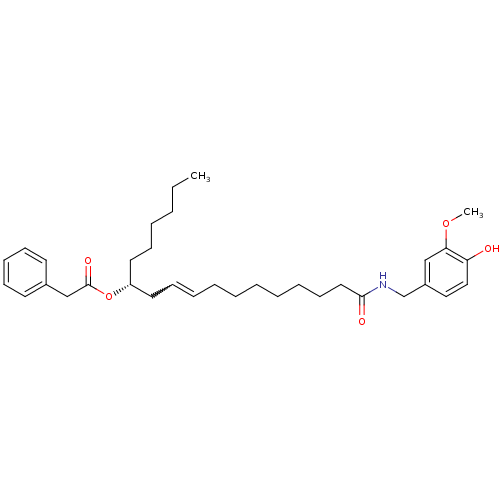 (PhAR) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Farmaceutiche e Farmacologiche Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 561-70 (2005) Article DOI: 10.1124/jpet.104.074864 BindingDB Entry DOI: 10.7270/Q2GB22NH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29081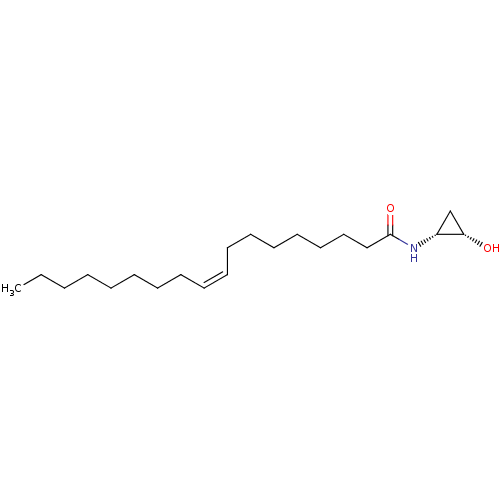 (cyclopropanolamide, 13b | cyclopropanolamide, rac-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 340 | -37.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29082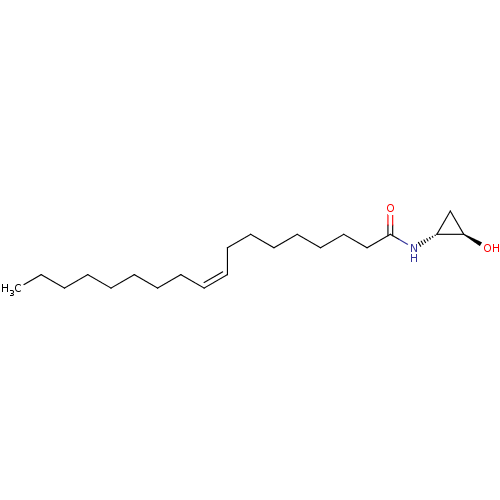 (cyclopropanolamide, 12b | cyclopropanolamide, rac-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 380 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052445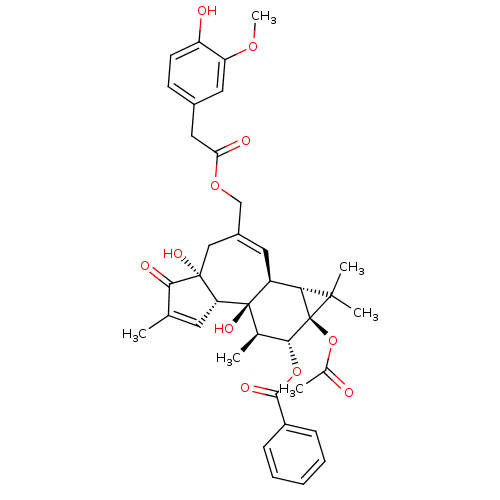 (Benzoic acid (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-9a-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052441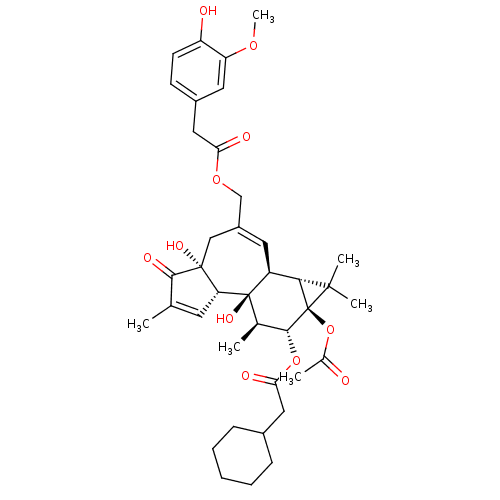 (CHEMBL317025 | Cyclohexyl-acetic acid (1aR,1bS,4aR...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM86539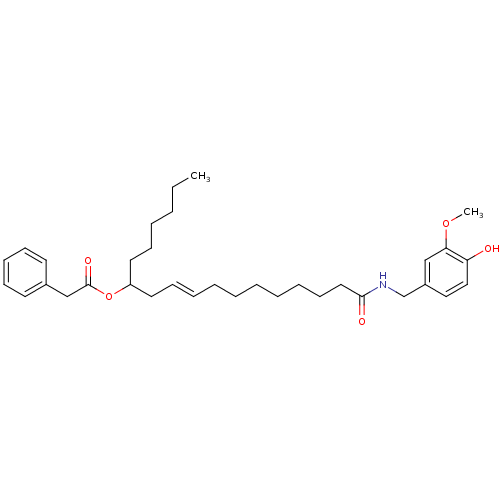 (PhAR, ent) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Farmaceutiche e Farmacologiche Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 561-70 (2005) Article DOI: 10.1124/jpet.104.074864 BindingDB Entry DOI: 10.7270/Q2GB22NH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50185034 (CHEMBL382444 | N-arachidonoyl-O-(2-hydroxyethyl)hy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Farmaceutiche Curated by ChEMBL | Assay Description Binding affinity to human recombinant CB1 receptor expressed in COS cells | J Med Chem 49: 2333-8 (2006) Article DOI: 10.1021/jm051240y BindingDB Entry DOI: 10.7270/Q2862G15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29082 (cyclopropanolamide, 12b | cyclopropanolamide, rac-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 570 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052440 (CHEMBL104647 | Phenyl-acetic acid (1aR,1bS,4aR,7aS...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]RTX binding from Vanilloid receptor of rat dorsal Root Ganglion (DRG) membranes | J Med Chem 39: 3123-31 (1996) Article DOI: 10.1021/jm960063l BindingDB Entry DOI: 10.7270/Q2VT1SRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29081 (cyclopropanolamide, 13b | cyclopropanolamide, rac-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -36.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM113765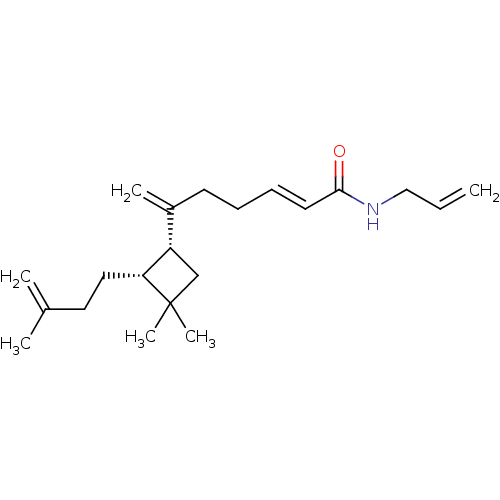 ((E)-N-Allyl-6-((1R,2R)-3,3-dimethyl-2-(3-methylbut...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 680 | -35.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bern , Bühlstrasse 28, CH-3012 Bern, Switzerland. | Assay Description In this assay, 18 ug of crude membrane expressing hCB1 or hCB2 receptors were re-suspended in 300 uL of binding buffer containing 50 mM Tris-HCl, 2.5... | ACS Chem Biol 9: 1499-507 (2014) Article DOI: 10.1021/cb500177c BindingDB Entry DOI: 10.7270/Q2T15295 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29081 (cyclopropanolamide, 13b | cyclopropanolamide, rac-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | -35.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29082 (cyclopropanolamide, 12b | cyclopropanolamide, rac-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | -35.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Universita del Piemonte Orientale | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 3001-9 (2009) Article DOI: 10.1021/jm900130m BindingDB Entry DOI: 10.7270/Q29G5K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50353094 (CHEMBL1823117) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` del Piemonte Orientale Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from human recombinant CB1 receptor expressed in HEK293 cells membrane incubated for 90 mins | J Nat Prod 74: 2019-22 (2011) Article DOI: 10.1021/np200500p BindingDB Entry DOI: 10.7270/Q2BR8SKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 692 total ) | Next | Last >> |