

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450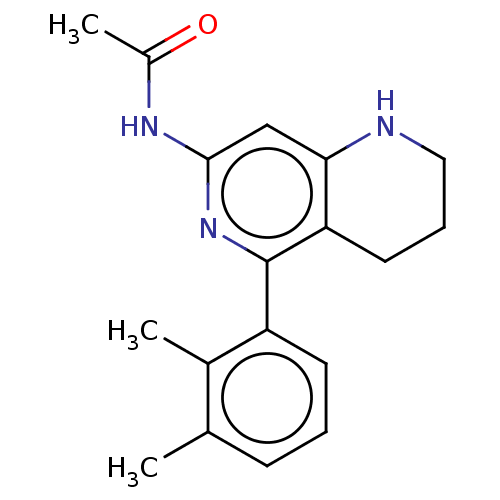 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511430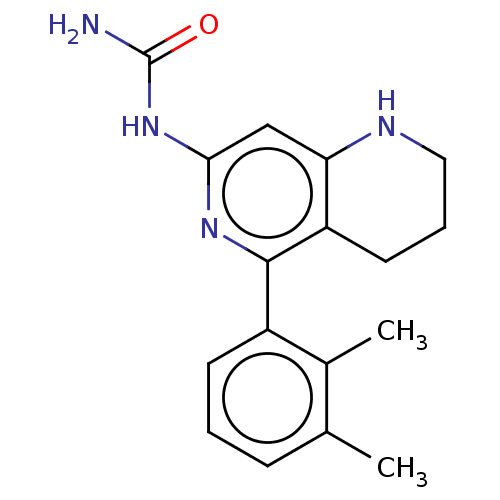 (CHEMBL4455353) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511431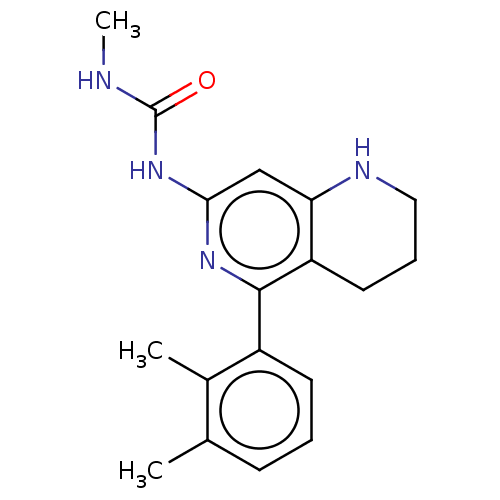 (CHEMBL4468099) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511453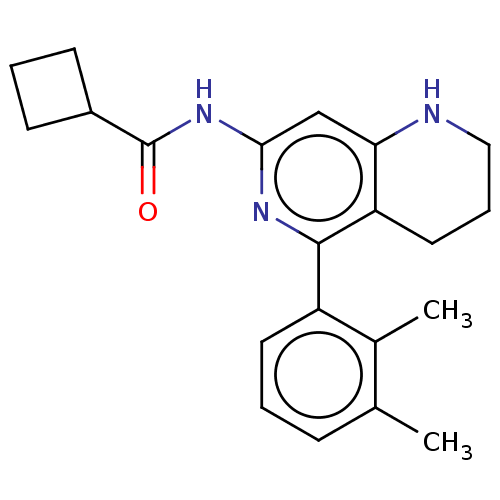 (CHEMBL4451394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511452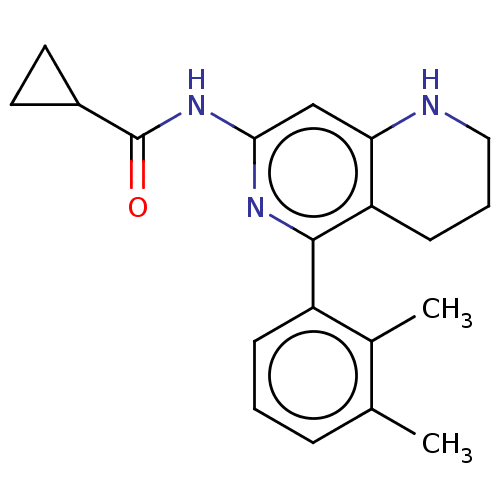 (CHEMBL4570266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511454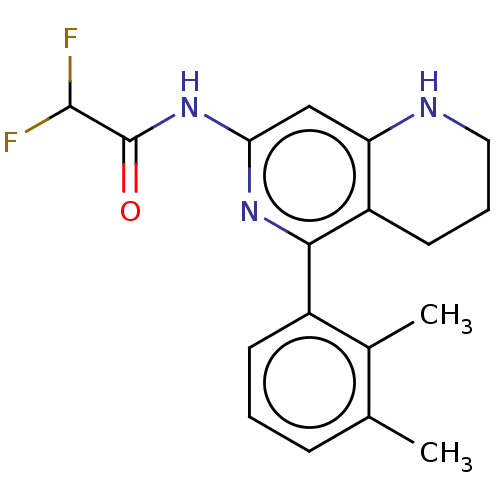 (CHEMBL4460228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511432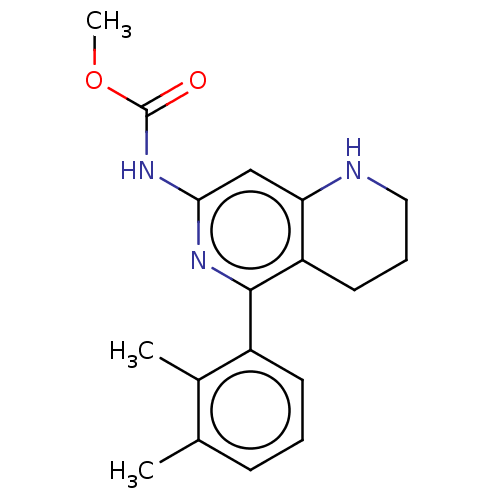 (CHEMBL4435945) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511466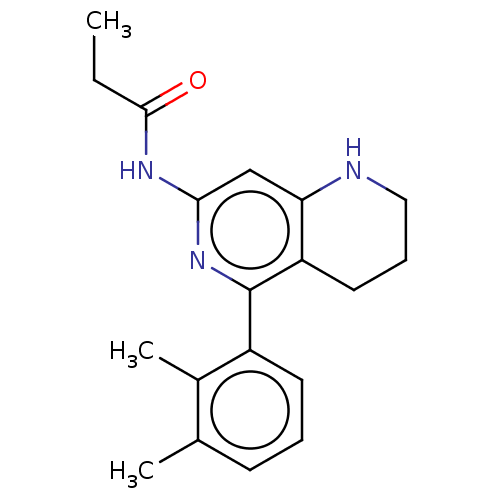 (CHEMBL4529335) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152144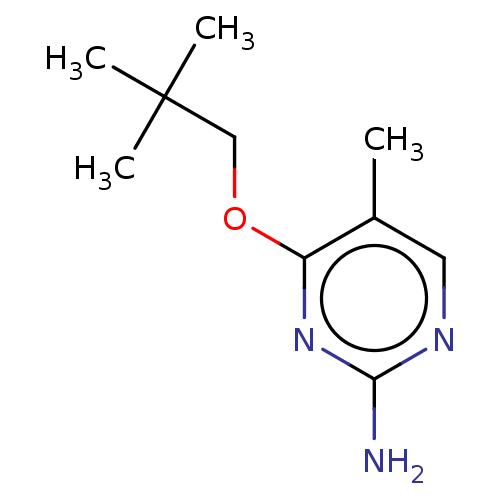 (CHEMBL3781661) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511448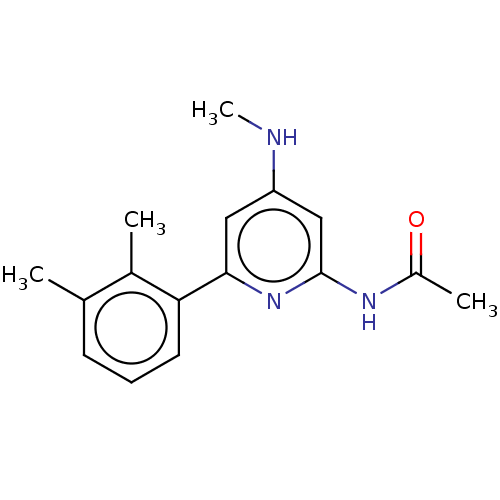 (CHEMBL4460446) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM187363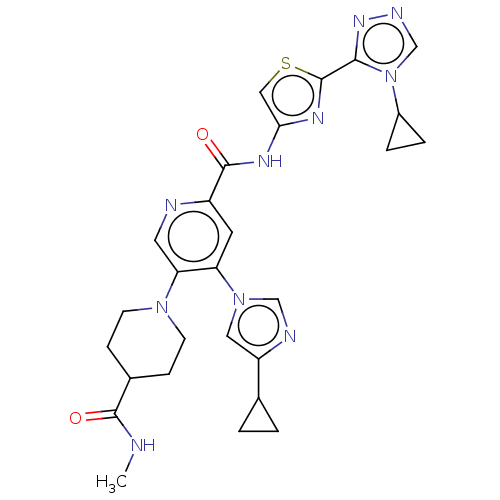 (US9169243, 11 | US9908875, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q22F7QRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM187363 (US9169243, 11 | US9908875, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | US Patent US9169243 (2015) BindingDB Entry DOI: 10.7270/Q2RF5ST3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511428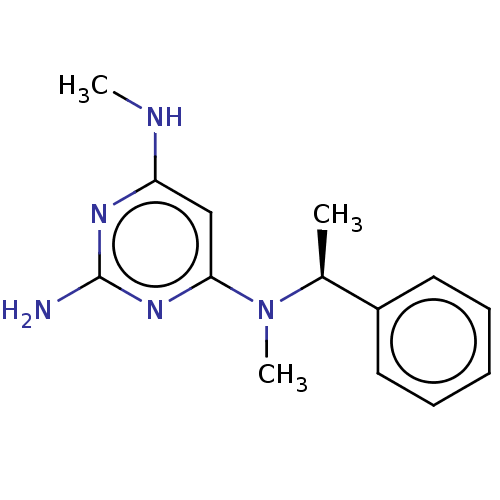 (CHEMBL4435983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50207351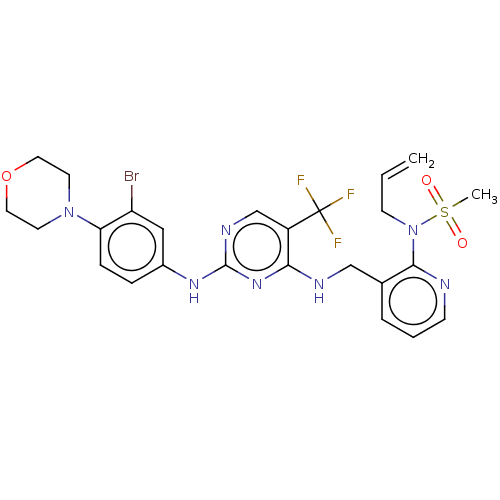 (CHEMBL3965256) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of NH2-terminal His6-tagged FAK kinase domain (410 to 689 residues) (unknown origin) expressed in baculovirus infected sf9 cells using p(G... | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50207359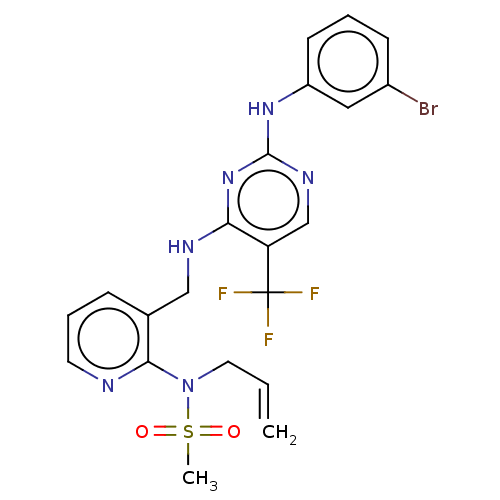 (CHEMBL3892377) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of NH2-terminal His6-tagged FAK kinase domain (410 to 689 residues) (unknown origin) expressed in baculovirus infected sf9 cells using p(G... | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511463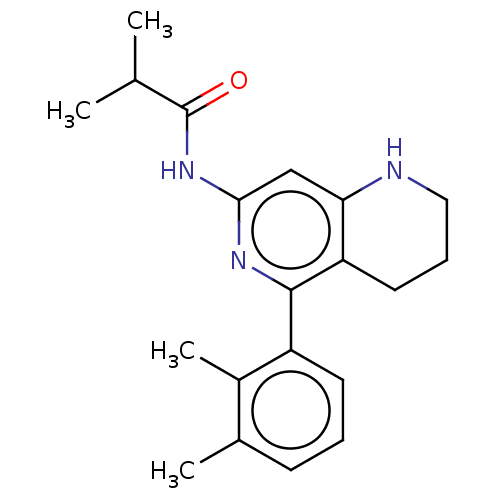 (CHEMBL4577002) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50207353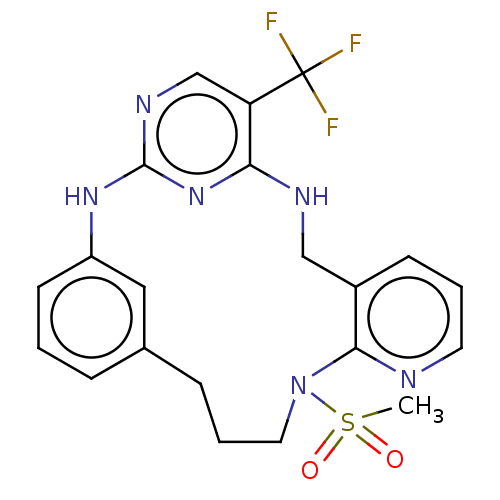 (CHEMBL3949755) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His6-tagged PYK2 expressed in baculovirus infected sf21 cells | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM162322 (US10307427, Compound 13 | US9051313, 13) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection (6.1). This is a competitive... | J Med Chem 52: 2188-91 (2009) BindingDB Entry DOI: 10.7270/Q2K64MD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511457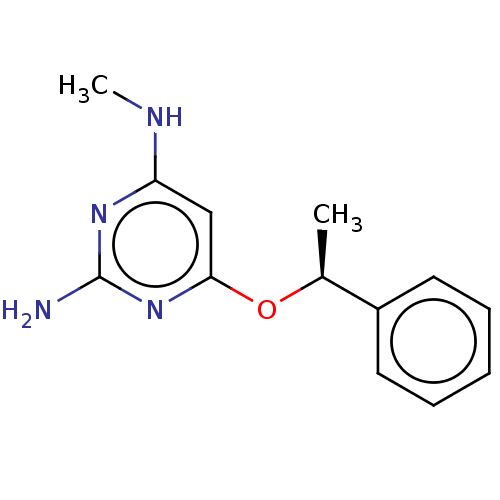 (CHEMBL4537820) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM162322 (US10307427, Compound 13 | US9051313, 13) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gildead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection (6.1). This is a competitive... | US Patent US9051313 (2015) BindingDB Entry DOI: 10.7270/Q21N7ZVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152125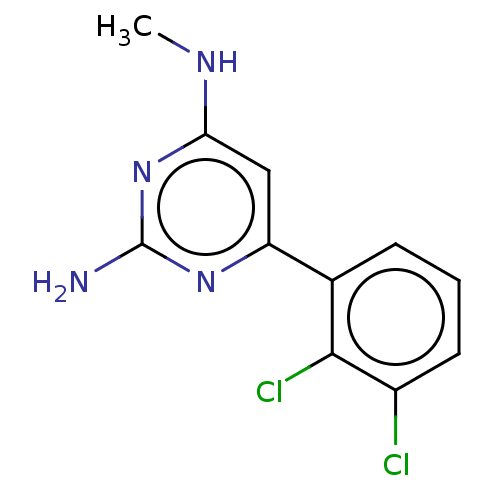 (CHEMBL3781316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using dGTP as substrate measured after 30 mins by mala... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511439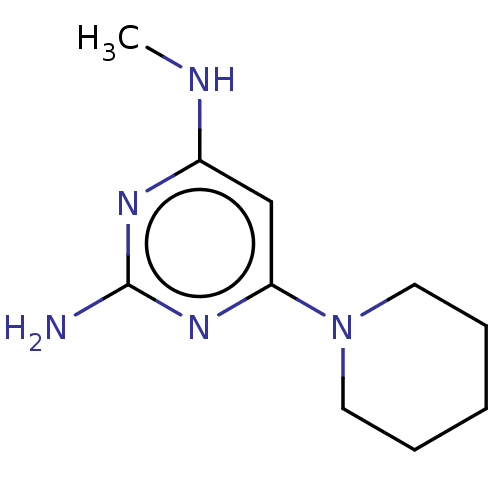 (CHEMBL4565830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511458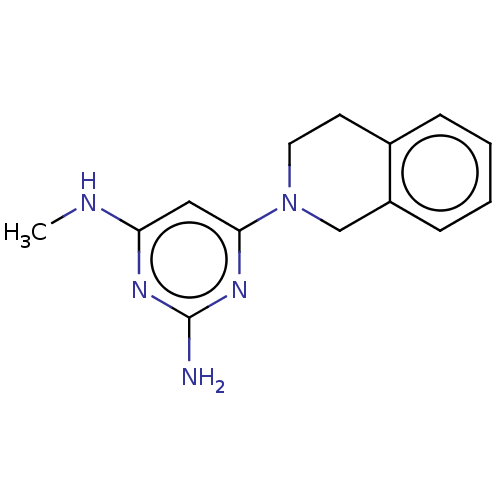 (CHEMBL4534555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50207349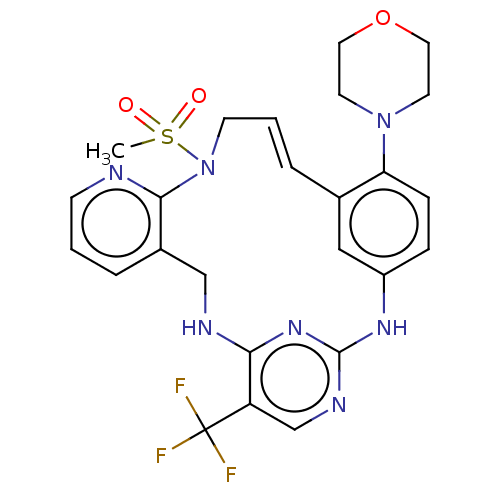 (CHEMBL3958414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His6-tagged PYK2 expressed in baculovirus infected sf21 cells | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM187354 (US9169243, 2 | US9908875, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q22F7QRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM187354 (US9169243, 2 | US9908875, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | US Patent US9169243 (2015) BindingDB Entry DOI: 10.7270/Q2RF5ST3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50207350 (CHEMBL3956954) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His6-tagged PYK2 expressed in baculovirus infected sf21 cells | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM187369 (US9169243, 17 | US9908875, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | US Patent US9169243 (2015) BindingDB Entry DOI: 10.7270/Q2RF5ST3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511441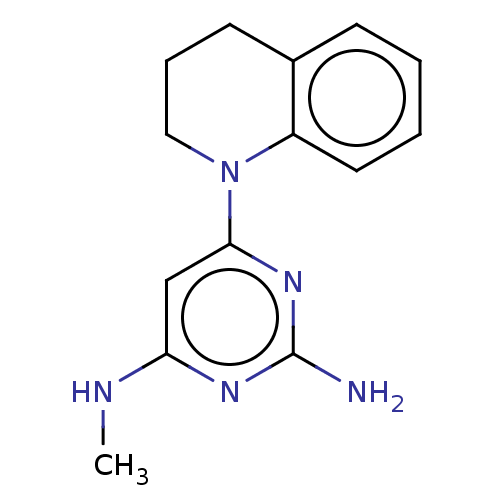 (CHEMBL4464305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM187369 (US9169243, 17 | US9908875, 17) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q22F7QRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM187385 (US9169243, 33 | US9908875, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | US Patent US9169243 (2015) BindingDB Entry DOI: 10.7270/Q2RF5ST3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM187385 (US9169243, 33 | US9908875, 33) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q22F7QRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50207353 (CHEMBL3949755) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of NH2-terminal His6-tagged FAK kinase domain (410 to 689 residues) (unknown origin) expressed in baculovirus infected sf9 cells using p(G... | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50207355 (CHEMBL3987106) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His6-tagged PYK2 expressed in baculovirus infected sf21 cells | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM187376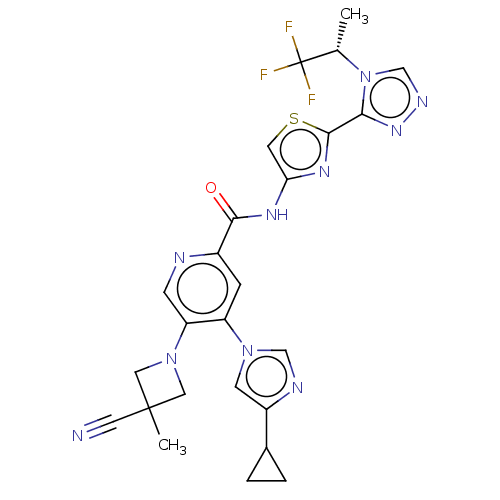 (US9169243, 24 | US9908875, 24) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q22F7QRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM187376 (US9169243, 24 | US9908875, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | US Patent US9169243 (2015) BindingDB Entry DOI: 10.7270/Q2RF5ST3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM162310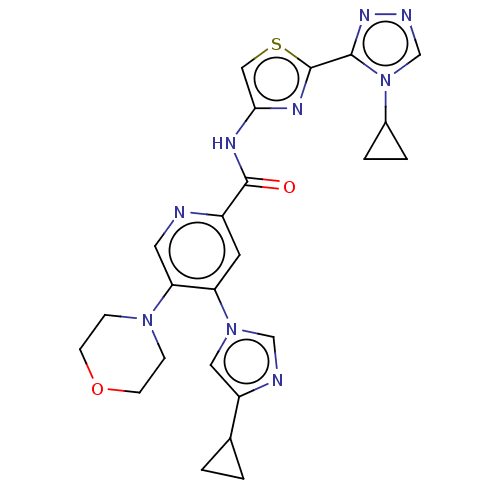 (US10307427, Compound 1 | US9051313, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a |
Gildead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection (6.1). This is a competitive... | US Patent US9051313 (2015) BindingDB Entry DOI: 10.7270/Q21N7ZVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM162310 (US10307427, Compound 1 | US9051313, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection (6.1). This is a competitive... | J Med Chem 52: 2188-91 (2009) BindingDB Entry DOI: 10.7270/Q2K64MD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM187384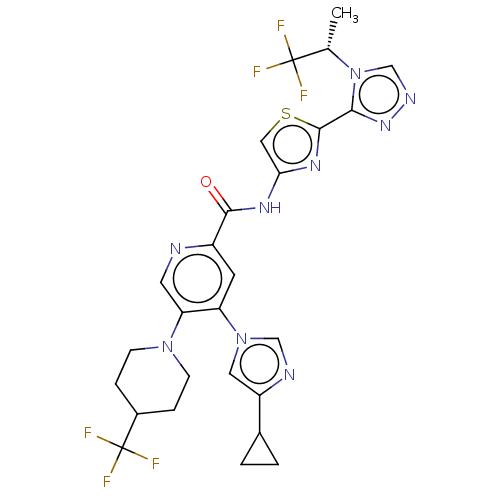 (US9169243, 32 | US9908875, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | US Patent US9169243 (2015) BindingDB Entry DOI: 10.7270/Q2RF5ST3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50207365 (CHEMBL3899046) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His6-tagged PYK2 expressed in baculovirus infected sf21 cells | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50318884 (CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible/competitive inhibition of NH2-terminal His6-tagged FAK kinase domain (410 to 689 residues) (unknown origin) expressed in baculovirus infec... | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM187384 (US9169243, 32 | US9908875, 32) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q22F7QRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM162311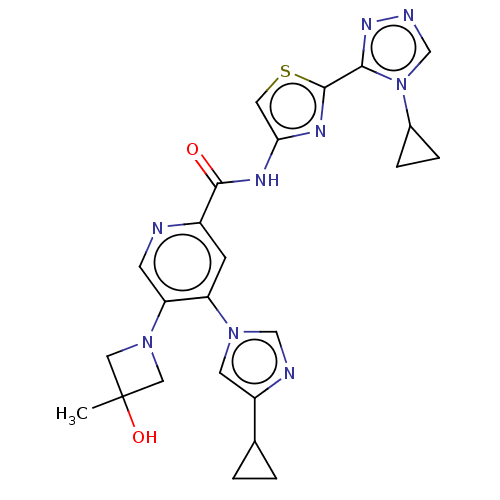 (US10307427, Compound 2 | US9051313, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Gildead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection (6.1). This is a competitive... | US Patent US9051313 (2015) BindingDB Entry DOI: 10.7270/Q21N7ZVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM162311 (US10307427, Compound 2 | US9051313, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection (6.1). This is a competitive... | J Med Chem 52: 2188-91 (2009) BindingDB Entry DOI: 10.7270/Q2K64MD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50207356 (CHEMBL3969464) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His6-tagged PYK2 expressed in baculovirus infected sf21 cells | Bioorg Med Chem Lett 26: 5926-5930 (2016) Article DOI: 10.1016/j.bmcl.2016.10.092 BindingDB Entry DOI: 10.7270/Q2ST7RTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM162313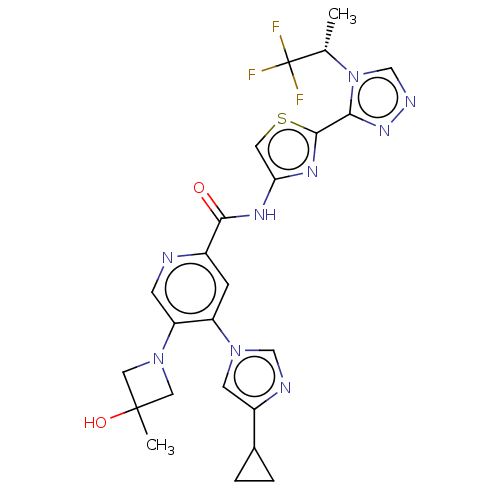 (US9051313, 4) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.69 | n/a | n/a | n/a | n/a | n/a | n/a |
Gildead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection (6.1). This is a competitive... | US Patent US9051313 (2015) BindingDB Entry DOI: 10.7270/Q21N7ZVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM394380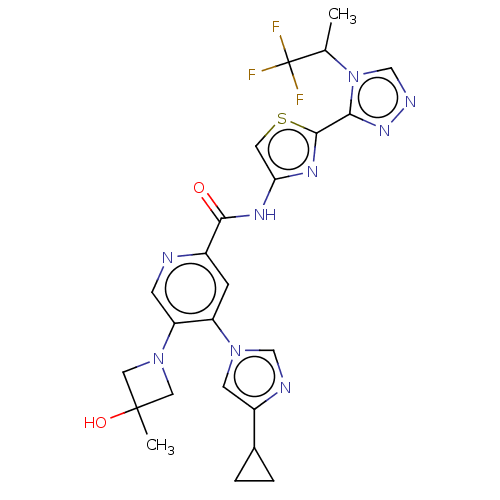 ((S)-4-(4-cyclopropyl-1H-imidazol-1-yl)-5-(3-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.69 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection (6.1). This is a competitive... | J Med Chem 52: 2188-91 (2009) BindingDB Entry DOI: 10.7270/Q2K64MD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM187386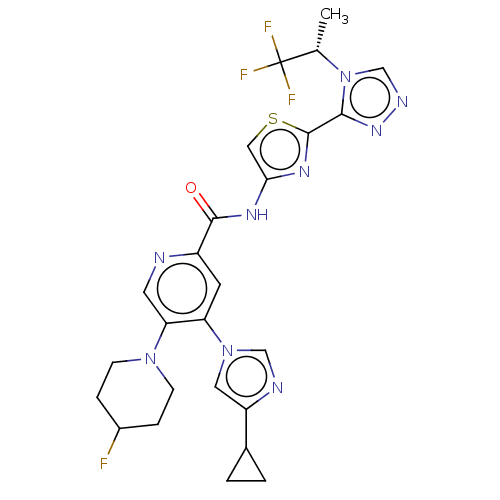 (US9169243, 34 | US9908875, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The assay measures the phosphorylation level of a biotinylated peptide substrate by the ASK1 kinase using HTRF detection. This is a competitive, time... | US Patent US9169243 (2015) BindingDB Entry DOI: 10.7270/Q2RF5ST3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 612 total ) | Next | Last >> |