

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50156495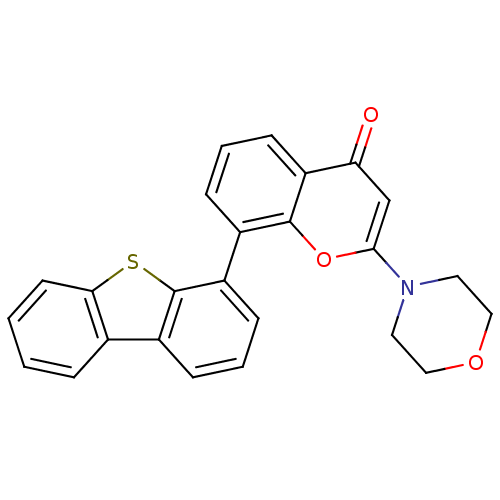 (8-(dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H-chro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Natural Sciences--Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against DNA-dependent protein kinase receptor | J Med Chem 48: 7829-46 (2005) Article DOI: 10.1021/jm050444b BindingDB Entry DOI: 10.7270/Q2Z31Z60 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM15234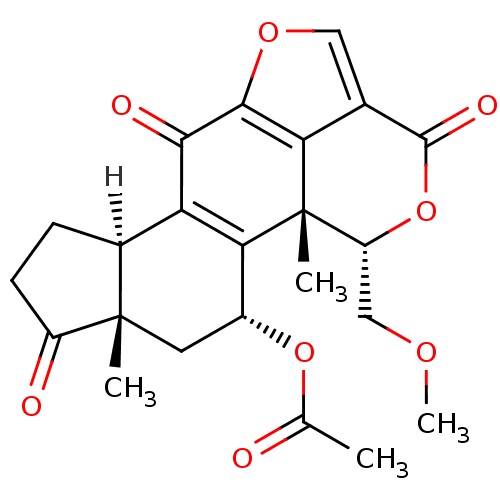 ((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description Inhibition of human recombinant p110 alpha Phosphatidylinositol 3-kinase | J Med Chem 48: 569-85 (2005) Article DOI: 10.1021/jm049526a BindingDB Entry DOI: 10.7270/Q2BK1BV9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM15234 ((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description Binding affinity for DNA dependent protein kinase isolated from HeLa cells; Range is 20-120 | J Med Chem 48: 569-85 (2005) Article DOI: 10.1021/jm049526a BindingDB Entry DOI: 10.7270/Q2BK1BV9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM12915 (2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description Binding affinity for Phosphatidylinositol-3-kinase isolated from HeLa cells; Range is 20-120 | J Med Chem 48: 569-85 (2005) Article DOI: 10.1021/jm049526a BindingDB Entry DOI: 10.7270/Q2BK1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM12915 (2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Newcastle Curated by ChEMBL | Assay Description Affinity for DNA-dependent protein kinase(DNA-PK) from HeLa cell extract | Bioorg Med Chem Lett 13: 3083-6 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM12915 (2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Competitive inhibition of DNA-PK (unknown origin) in the presence of ATP | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50319926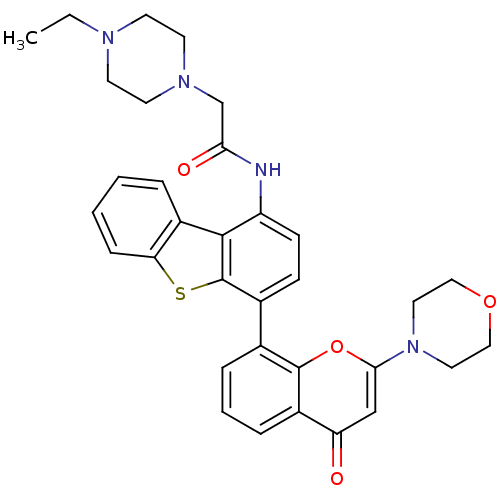 (2-(4-ethylpiperazin-1-yl)-N-(4-(2-morpholino-4-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of PI-3K delta (unknown origin) | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174595 (US9102670, 14a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174368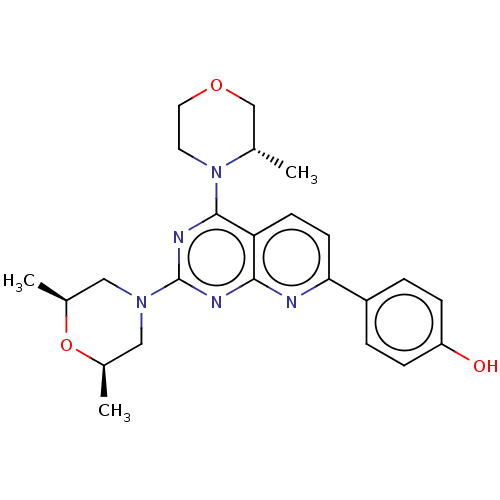 (US9102670, 1cg) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174414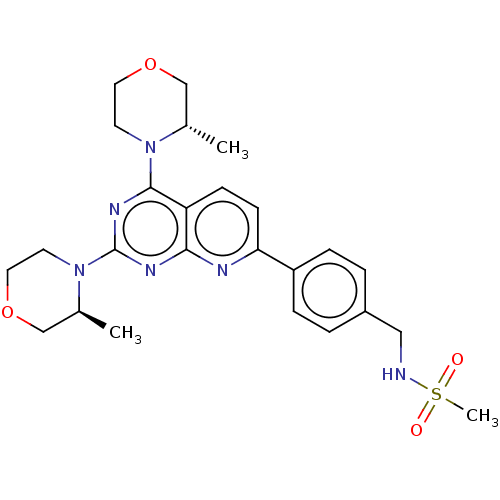 (US9102670, 1du) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50319926 (2-(4-ethylpiperazin-1-yl)-N-(4-(2-morpholino-4-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of PI-3K beta (unknown origin) | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Mus musculus (Mouse)) | BDBM27566 (4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
KuDOS Pharmaceuticals Ltd. | Assay Description A FlashPlate scintillation proximity assay has been used to identify inhibitors of PARP-2. PARP-2 protein (recombinant) was bound down by a PARP-2 sp... | J Med Chem 51: 6581-91 (2008) Article DOI: 10.1021/jm8001263 BindingDB Entry DOI: 10.7270/Q2BP014T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174467 (US9102670, 4a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174288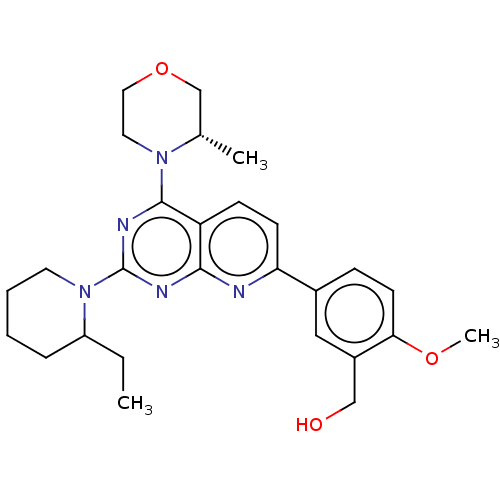 (US9102670, 1c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description For mTOR enzyme activity assays, mTOR protein was isolated from HeLa cell cytoplasmic extract by immunoprecipitation, and activity determined essenti... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174287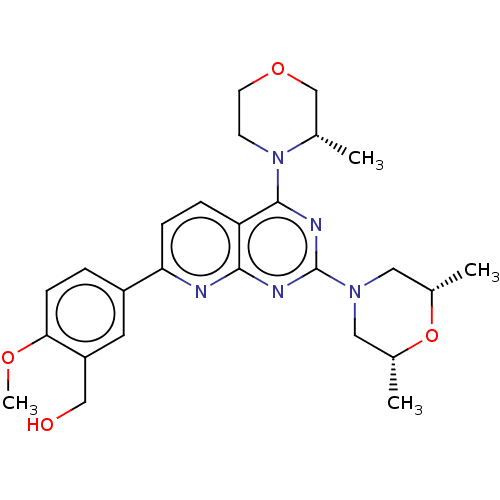 (US9102670, 1b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.85 | n/a | n/a | n/a | n/a | n/a | n/a |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description For mTOR enzyme activity assays, mTOR protein was isolated from HeLa cell cytoplasmic extract by immunoprecipitation, and activity determined essenti... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174471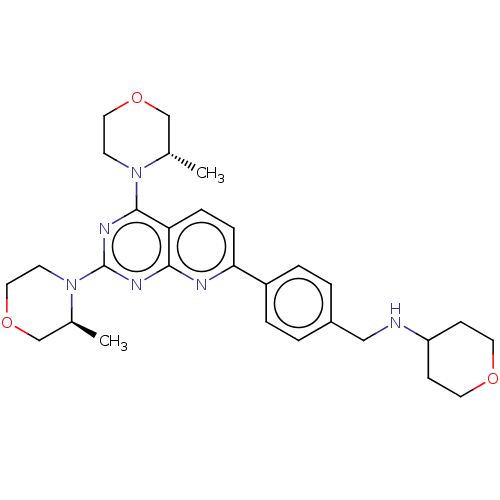 (US9102670, 4e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174472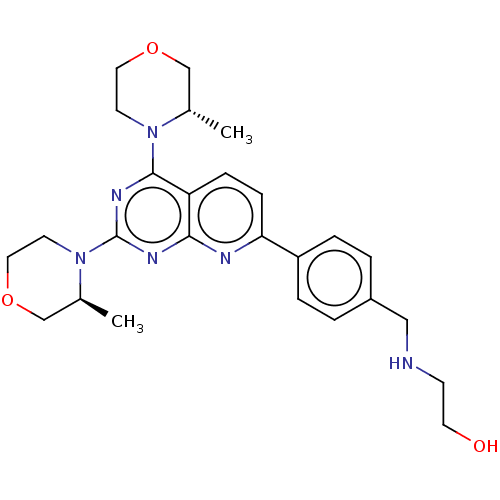 (US9102670, 4f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description For mTOR enzyme activity assays, mTOR protein was isolated from HeLa cell cytoplasmic extract by immunoprecipitation, and activity determined essenti... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27570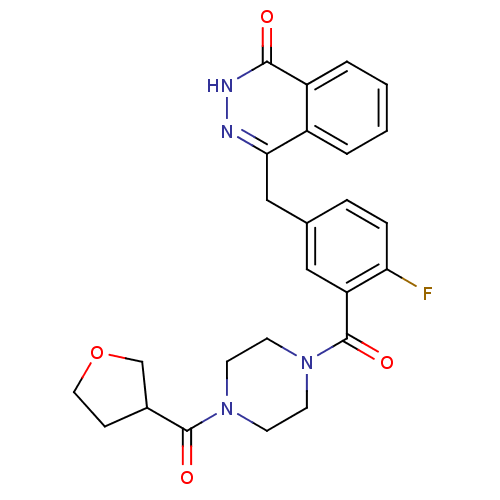 (4-[(4-fluoro-3-{[4-(oxolan-3-ylcarbonyl)piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
KuDOS Pharmaceuticals Ltd. | Assay Description A FlashPlate scintillation proximity assay has been developed to identify inhibitors of PARP-1. The mechanism of action of the assay requires the bin... | J Med Chem 51: 6581-91 (2008) Article DOI: 10.1021/jm8001263 BindingDB Entry DOI: 10.7270/Q2BP014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50439946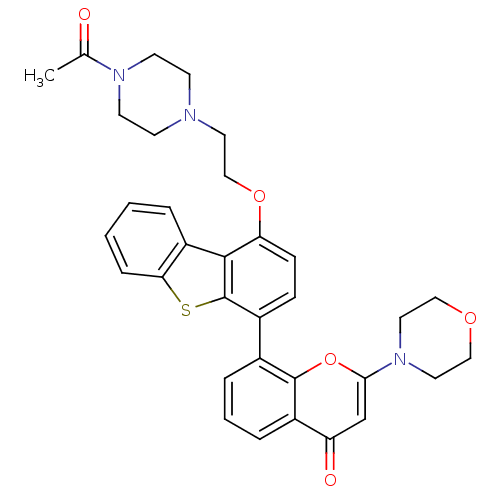 (CHEMBL2420287) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of human DNA-PK using GST-tagged p53N66 as substrate after 1 hr by ELISA-based chemiluminiscence assay | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50439892 (CHEMBL2420289) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of human DNA-PK using GST-tagged p53N66 as substrate after 1 hr by ELISA-based chemiluminiscence assay | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50439893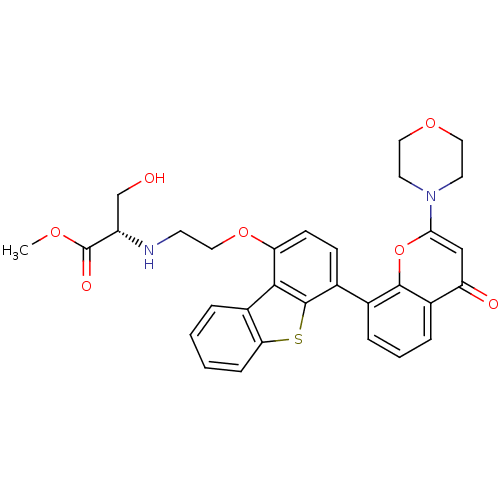 (CHEMBL2420254) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of human DNA-PK using GST-tagged p53N66 as substrate after 1 hr by ELISA-based chemiluminiscence assay | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27567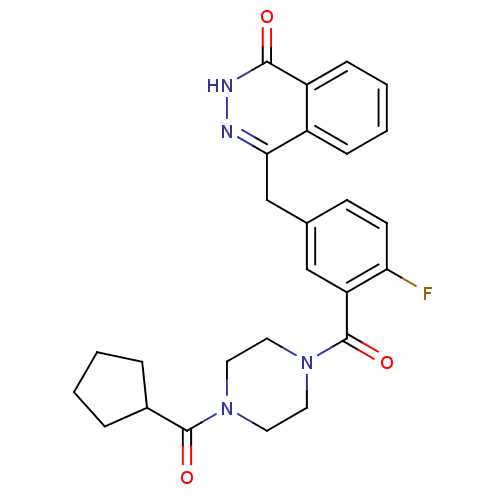 (4-({3-[(4-cyclopentanecarbonylpiperazin-1-yl)carbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
KuDOS Pharmaceuticals Ltd. | Assay Description A FlashPlate scintillation proximity assay has been developed to identify inhibitors of PARP-1. The mechanism of action of the assay requires the bin... | J Med Chem 51: 6581-91 (2008) Article DOI: 10.1021/jm8001263 BindingDB Entry DOI: 10.7270/Q2BP014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27546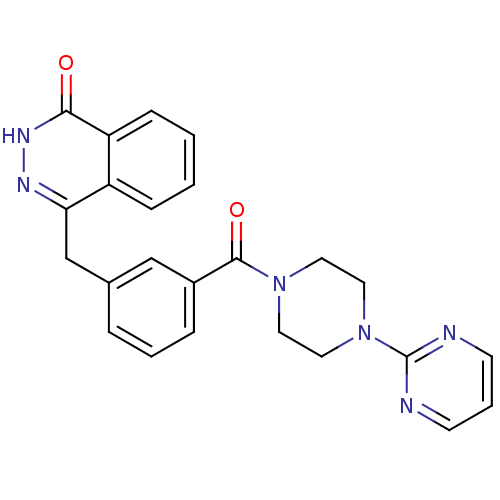 (4-[(3-{[4-(pyrimidin-2-yl)piperazin-1-yl]carbonyl}...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
KuDOS Pharmaceuticals Ltd. | Assay Description A FlashPlate scintillation proximity assay has been developed to identify inhibitors of PARP-1. The mechanism of action of the assay requires the bin... | J Med Chem 51: 6581-91 (2008) Article DOI: 10.1021/jm8001263 BindingDB Entry DOI: 10.7270/Q2BP014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27542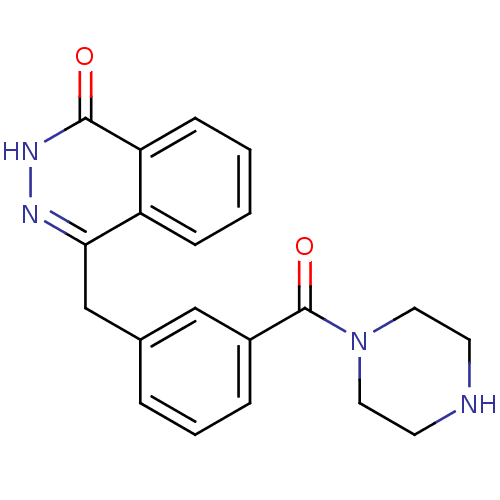 (4-(3-(piperazine-1-carbonyl)benzyl)phthalazin-1(2H...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
KuDOS Pharmaceuticals Ltd | Assay Description A FlashPlate scintillation proximity assay has been developed to identify inhibitors of PARP-1. The mechanism of action of the assay requires the bin... | Bioorg Med Chem Lett 18: 3942-5 (2008) Article DOI: 10.1016/j.bmcl.2008.06.025 BindingDB Entry DOI: 10.7270/Q2FF3QPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27542 (4-(3-(piperazine-1-carbonyl)benzyl)phthalazin-1(2H...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
KuDOS Pharmaceuticals Ltd. | Assay Description A FlashPlate scintillation proximity assay has been developed to identify inhibitors of PARP-1. The mechanism of action of the assay requires the bin... | J Med Chem 51: 6581-91 (2008) Article DOI: 10.1021/jm8001263 BindingDB Entry DOI: 10.7270/Q2BP014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27545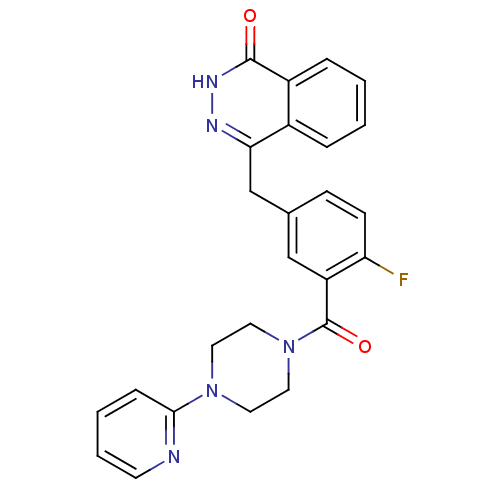 (4-[(4-fluoro-3-{[4-(pyridin-2-yl)piperazin-1-yl]ca...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
KuDOS Pharmaceuticals Ltd. | Assay Description A FlashPlate scintillation proximity assay has been developed to identify inhibitors of PARP-1. The mechanism of action of the assay requires the bin... | J Med Chem 51: 6581-91 (2008) Article DOI: 10.1021/jm8001263 BindingDB Entry DOI: 10.7270/Q2BP014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174293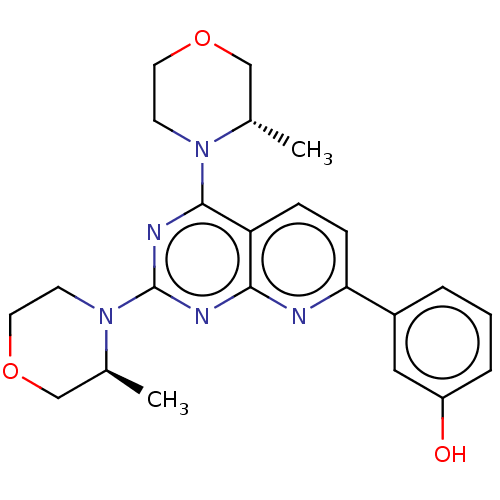 (US9102670, 1h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174289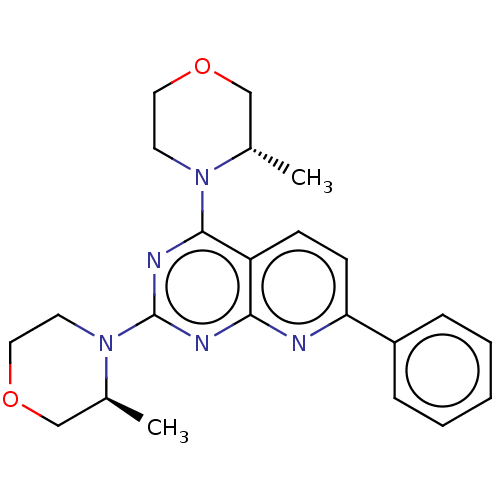 (US9102670, 1d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description For mTOR enzyme activity assays, mTOR protein was isolated from HeLa cell cytoplasmic extract by immunoprecipitation, and activity determined essenti... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174413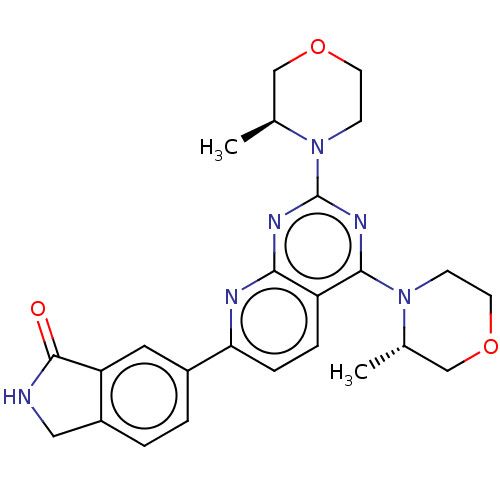 (US9102670, 1dt) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174686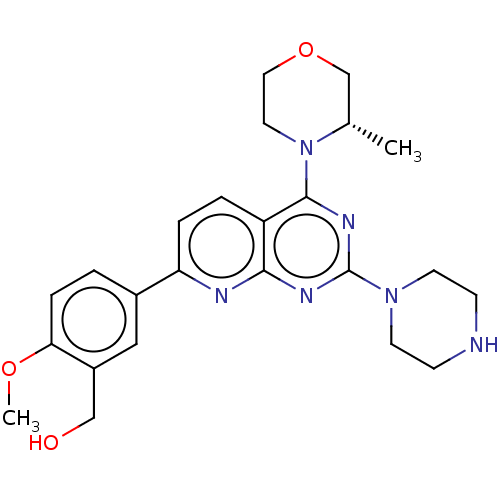 (US9102670, 18ca) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174347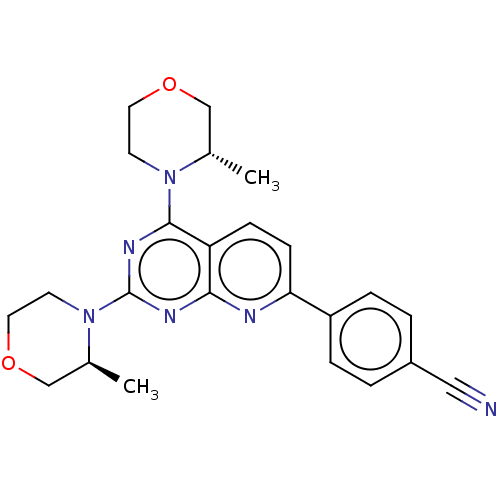 (US9102670, 1bl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174472 (US9102670, 4f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27547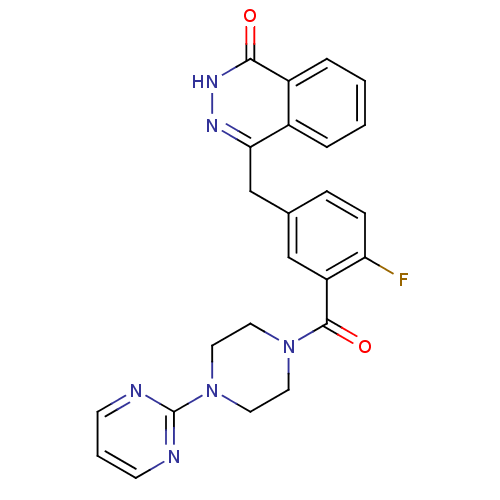 (4-[(4-fluoro-3-{[4-(pyrimidin-2-yl)piperazin-1-yl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
KuDOS Pharmaceuticals Ltd. | Assay Description A FlashPlate scintillation proximity assay has been developed to identify inhibitors of PARP-1. The mechanism of action of the assay requires the bin... | J Med Chem 51: 6581-91 (2008) Article DOI: 10.1021/jm8001263 BindingDB Entry DOI: 10.7270/Q2BP014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27569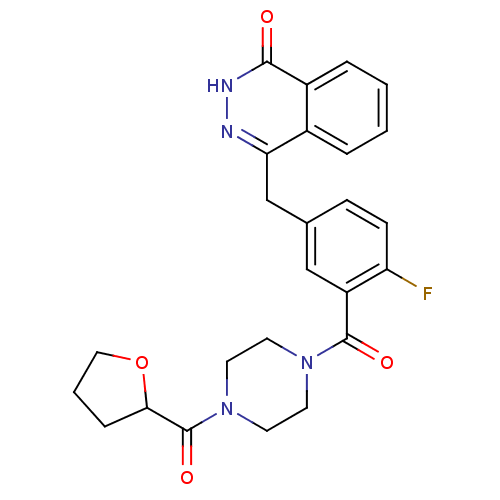 (4-[(4-fluoro-3-{[4-(oxolan-2-ylcarbonyl)piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
KuDOS Pharmaceuticals Ltd. | Assay Description A FlashPlate scintillation proximity assay has been developed to identify inhibitors of PARP-1. The mechanism of action of the assay requires the bin... | J Med Chem 51: 6581-91 (2008) Article DOI: 10.1021/jm8001263 BindingDB Entry DOI: 10.7270/Q2BP014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50439957 (CHEMBL2420255) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of human DNA-PK using GST-tagged p53N66 as substrate after 1 hr by ELISA-based chemiluminiscence assay | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50439880 (CHEMBL2420253) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of human DNA-PK using GST-tagged p53N66 as substrate after 1 hr by ELISA-based chemiluminiscence assay | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174712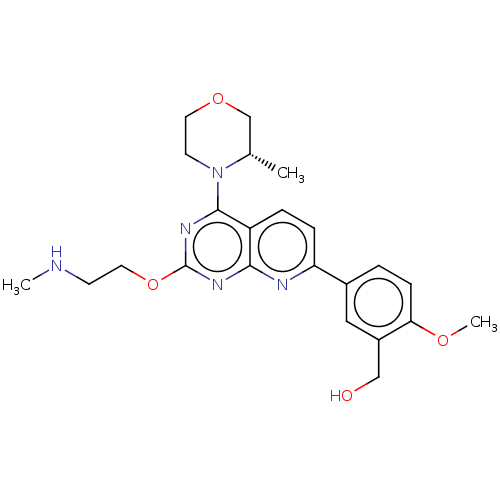 (US9102670, 18db) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50429706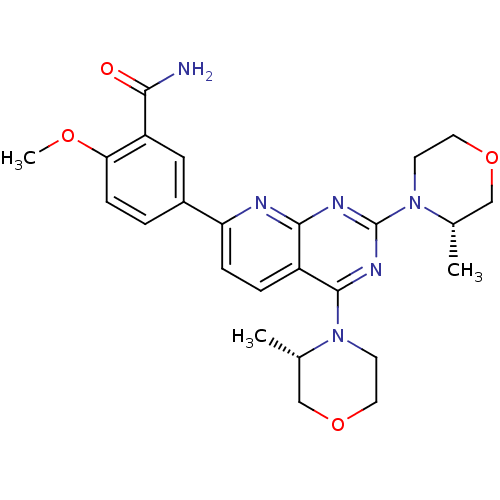 (CHEMBL2336320 | US9102670, 14b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174418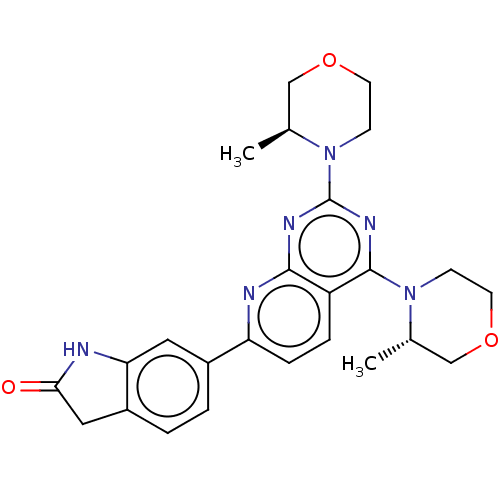 (US9102670, 1dy) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174514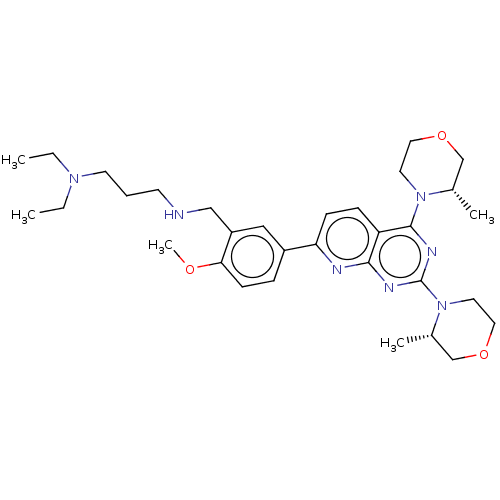 (US9102670, 4av) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165496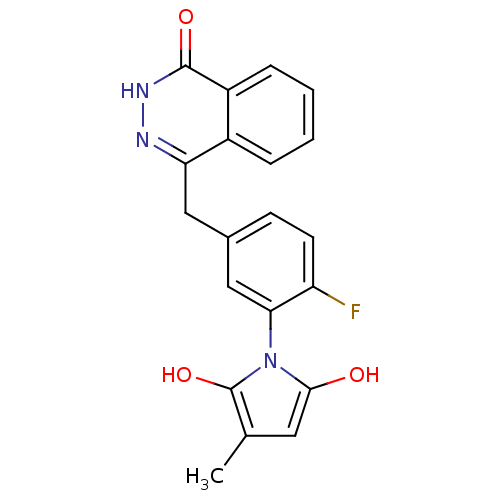 (1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
KuDOS Horsham Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against human poly (ADP-ribose) polymerase 1 (PARP-1) | Bioorg Med Chem Lett 15: 2235-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.026 BindingDB Entry DOI: 10.7270/Q2QV3M0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50319926 (2-(4-ethylpiperazin-1-yl)-N-(4-(2-morpholino-4-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of PI-3K alpha (unknown origin) | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50439870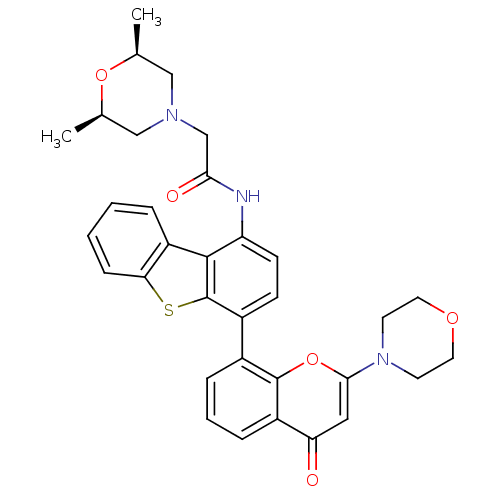 (CHEMBL2420439) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of human DNA-PK using GST-tagged p53N66 as substrate after 1 hr by ELISA-based chemiluminiscence assay | J Med Chem 56: 6386-401 (2013) Article DOI: 10.1021/jm400915j BindingDB Entry DOI: 10.7270/Q2RF5WFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27563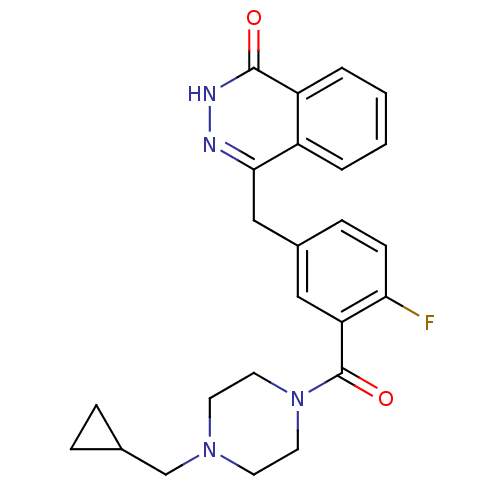 (4-[(3-{[4-(cyclopropylmethyl)piperazin-1-yl]carbon...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
KuDOS Pharmaceuticals Ltd. | Assay Description A FlashPlate scintillation proximity assay has been developed to identify inhibitors of PARP-1. The mechanism of action of the assay requires the bin... | J Med Chem 51: 6581-91 (2008) Article DOI: 10.1021/jm8001263 BindingDB Entry DOI: 10.7270/Q2BP014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165488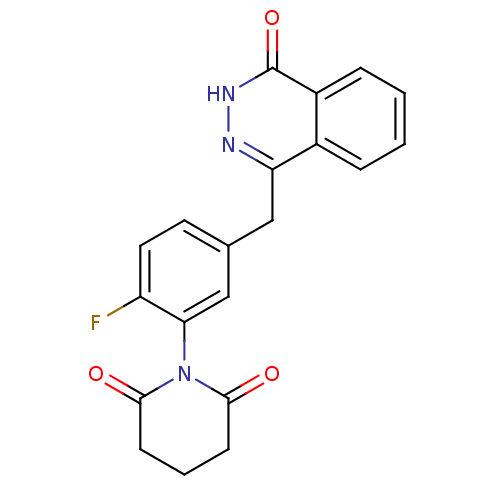 (1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
KuDOS Horsham Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against human poly (ADP-ribose) polymerase 1 (PARP-1) | Bioorg Med Chem Lett 15: 2235-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.026 BindingDB Entry DOI: 10.7270/Q2QV3M0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase theta (Homo sapiens) | BDBM50591272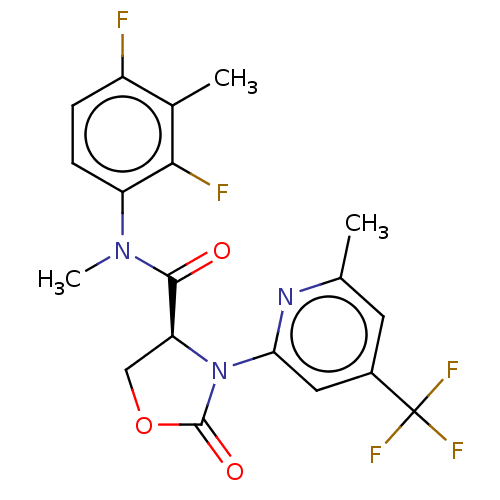 (CHEMBL5208956) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01142 BindingDB Entry DOI: 10.7270/Q2Z03D46 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174602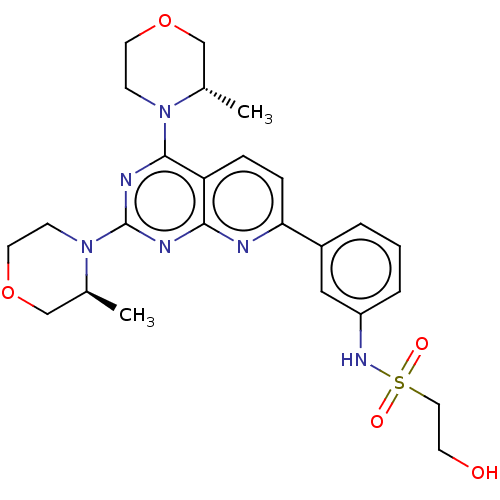 (US9102670, 15a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174290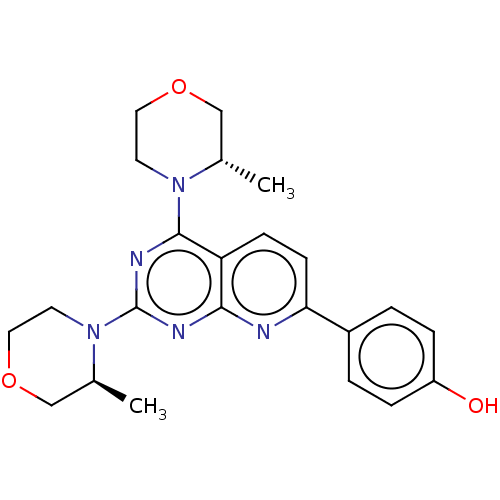 (US9102670, 1e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174360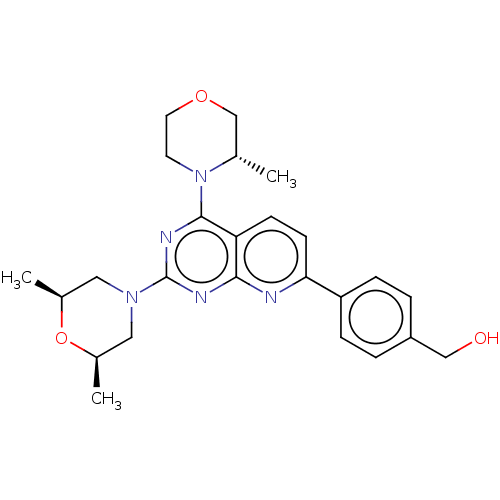 (US9102670, 1by) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174439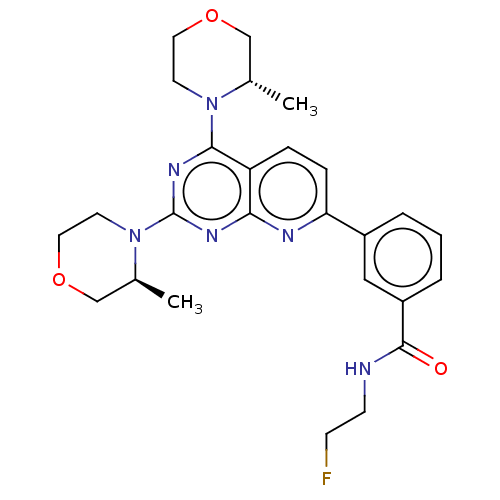 (US9102670, 3e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1248 total ) | Next | Last >> |