

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17461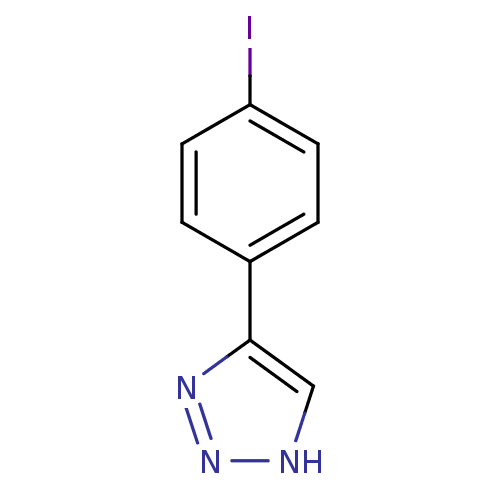 (1,2,3-triazole analogue, 17 | 5-(4-iodophenyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17468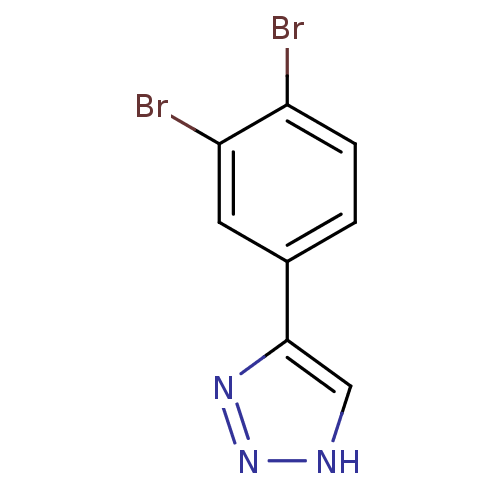 (1,2,3-triazole analogue, 24 | 5-(3,4-dibromophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17460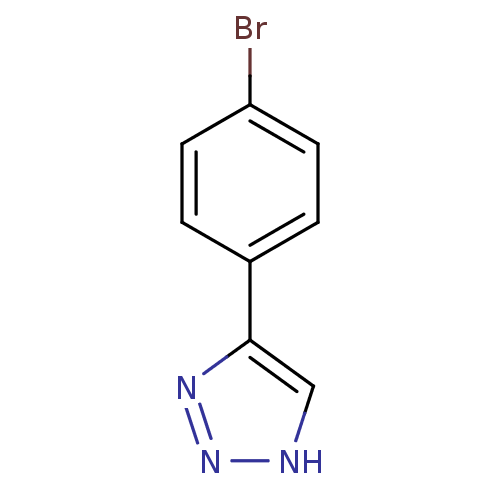 (1,2,3-triazole analogue, 16 | 5-(4-bromophenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17474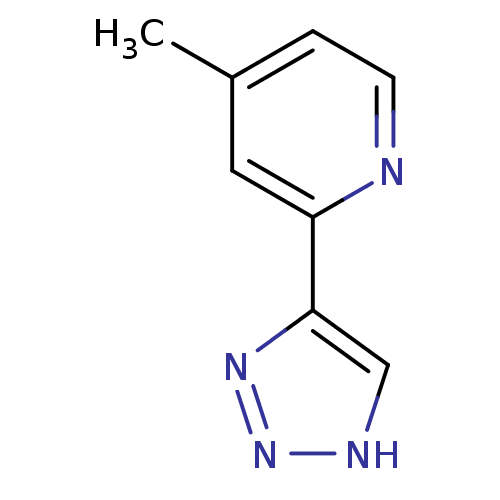 (1,2,3-triazole analogue, 30 | 4-methyl-2-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17467 (1,2,3-triazole analogue, 23 | 5-(3-bromophenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17459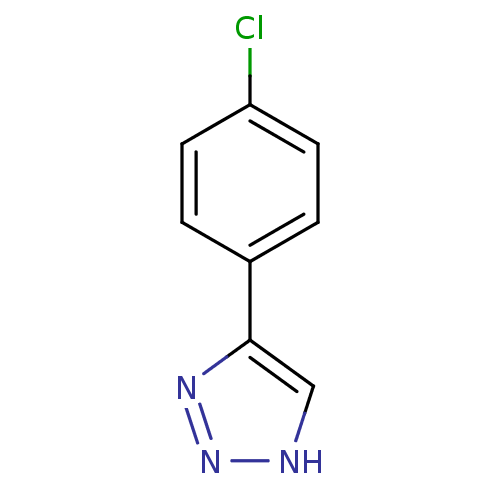 (1,2,3-triazole analogue, 15 | 5-(4-chlorophenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17473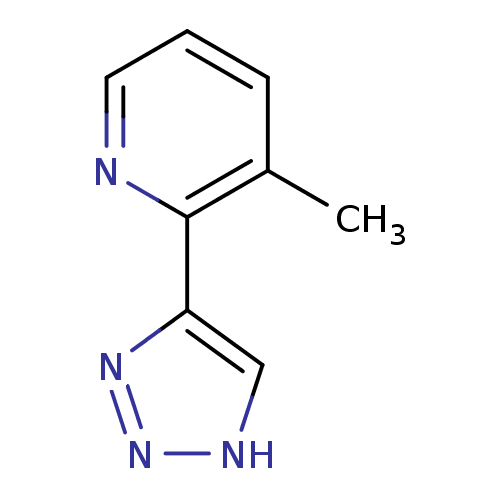 (1,2,3-triazole analogue, 29 | 3-methyl-2-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17475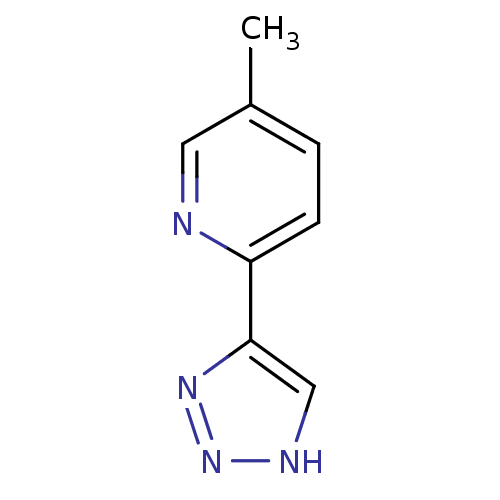 (1,2,3-triazole analogue, 31 | 5-methyl-2-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17469 (1,2,3-triazole analogue, 25 | 5-(1-benzofuran-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17447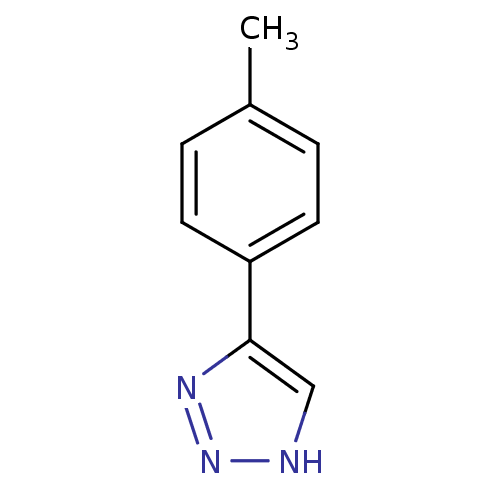 (1,2,3-triazole analogue, 3 | 5-(4-methylphenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17466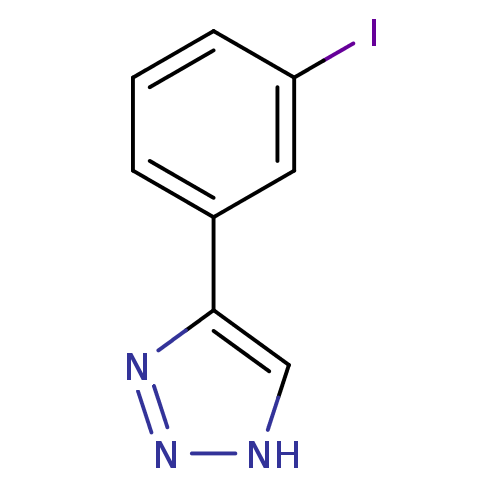 (1,2,3-triazole analogue, 22 | 5-(3-iodophenyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17462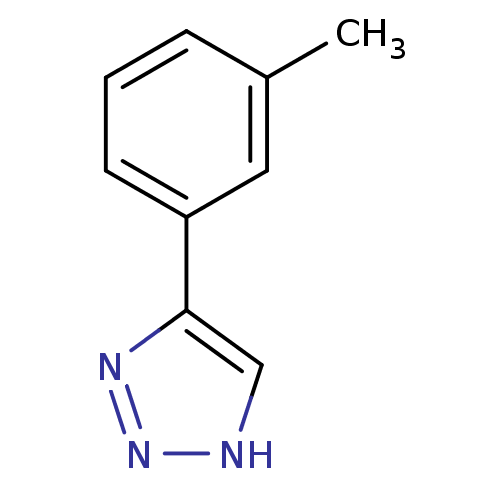 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 18 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17465 (1,2,3-triazole analogue, 21 | N-benzyl-3-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17455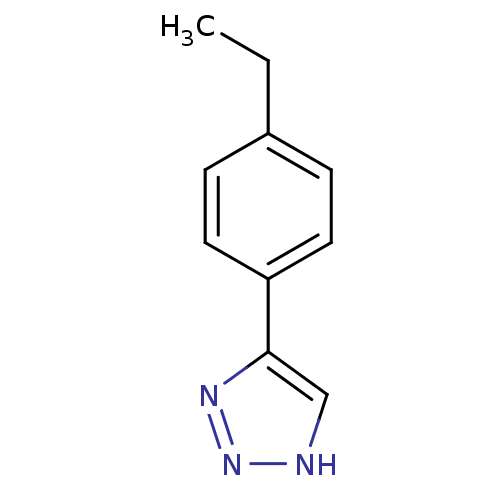 (1,2,3-triazole analogue, 11 | 5-(4-ethylphenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17470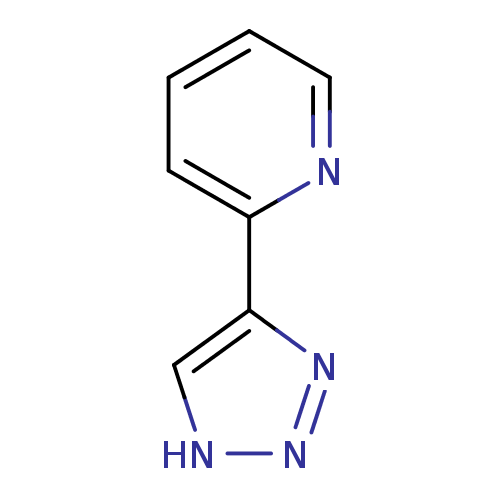 (1,2,3-triazole analogue, 26 | 2-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17449 (1,2,3-triazole analogue, 5 | 1H,4H-indeno[1,2-d][1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 52 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17476 (1,2,3-triazole analogue, 32 | 2-methyl-6-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 52 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17448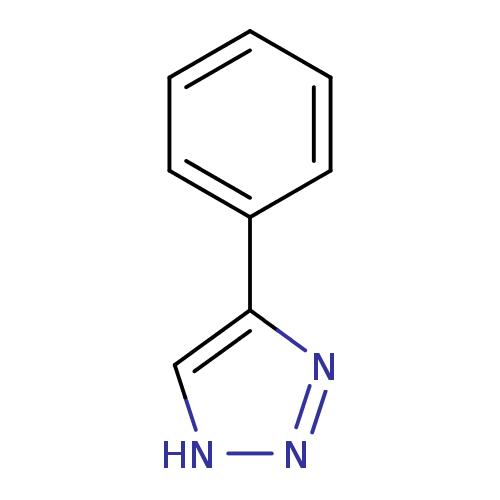 (1,2,3-triazole analogue, 4 | 5-phenyl-1H-1,2,3-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17452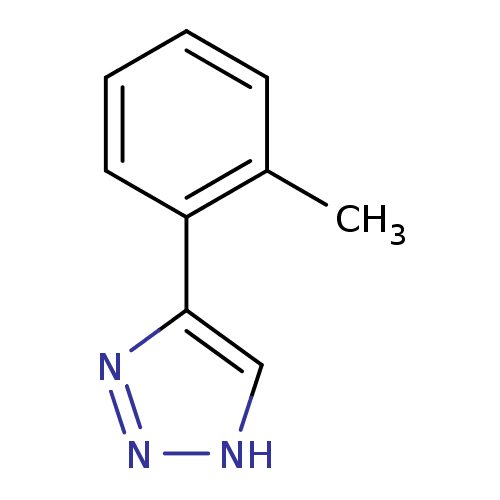 (1,2,3-triazole analogue, 8 | 5-(2-methylphenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 112 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17453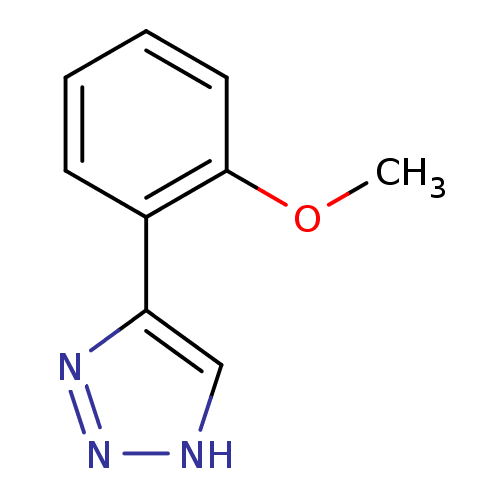 (1,2,3-triazole analogue, 9 | 5-(2-methoxyphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17471 (1,2,3-triazole analogue, 27 | 3-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 260 | -37.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17450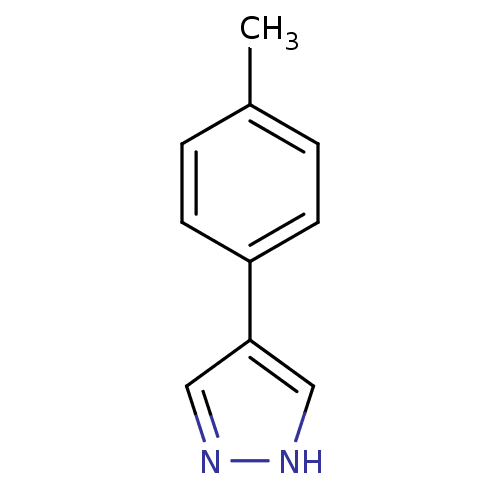 (4-(4-methylphenyl)-1H-pyrazole | pyrazole analogue...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17464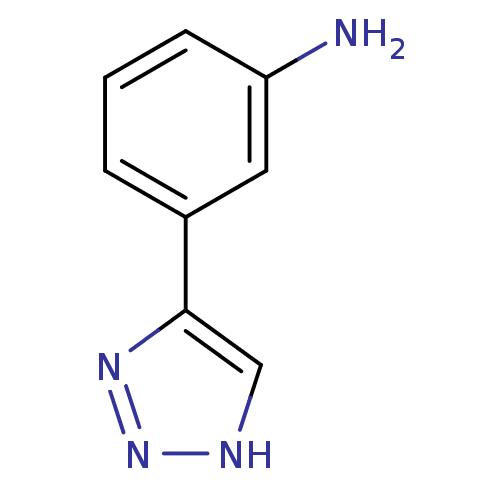 (1,2,3-triazole analogue, 20 | 3-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17463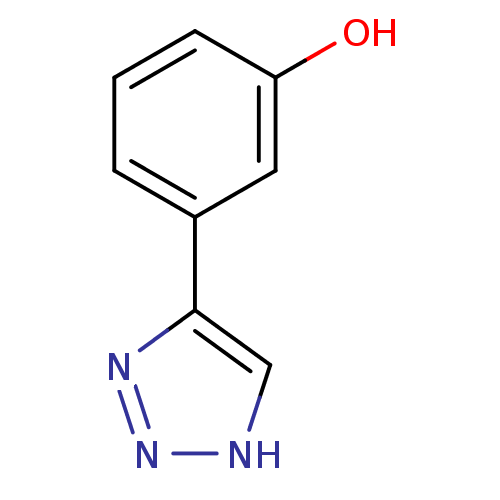 (1,2,3-triazole analogue, 19 | 3-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17458 (1,2,3-triazole analogue, 14 | 4-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 337 | -36.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17456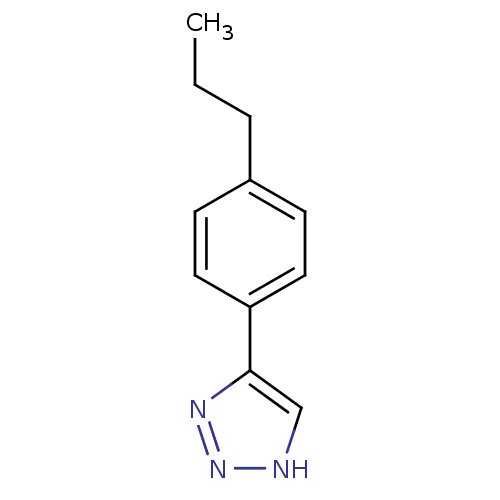 (1,2,3-triazole analogue, 12 | 5-(4-propylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | >-33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17457 (1,2,3-triazole analogue, 13 | 4-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17454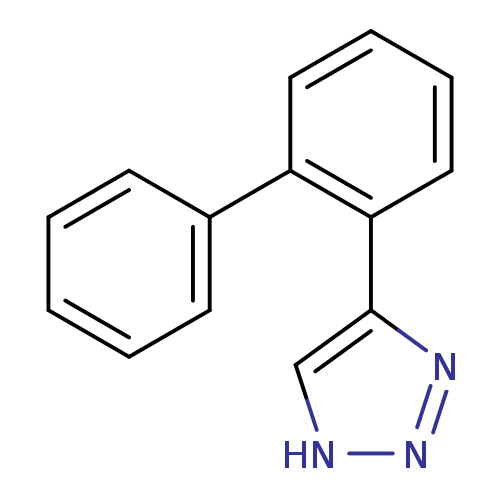 (1,2,3-triazole analogue, 10 | 5-(2-phenylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >1.00E+3 | >-33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17472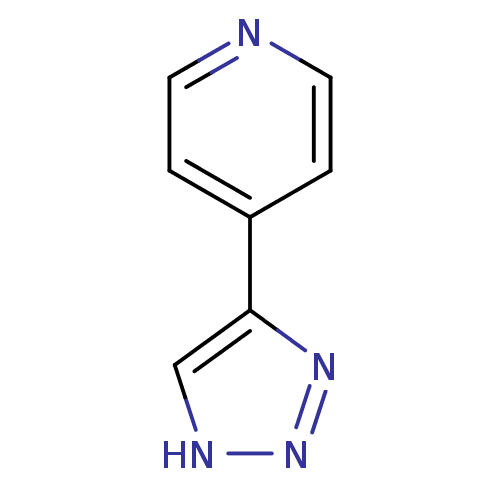 (1,2,3-triazole analogue, 28 | 4-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | >1.00E+3 | >-33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50520994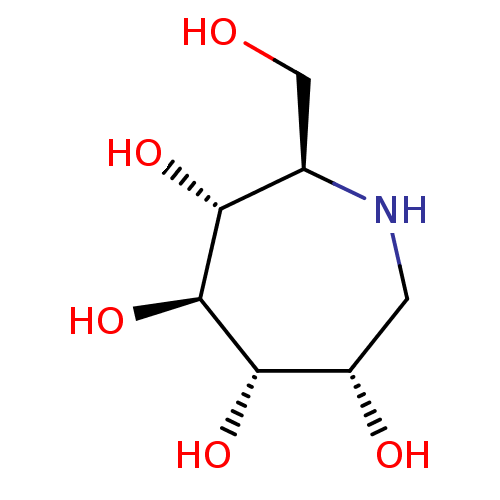 (CHEMBL4529797) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of green coffee bean alpha-galactosidase | Eur J Med Chem 162: 465-494 (2019) Article DOI: 10.1016/j.ejmech.2018.11.031 BindingDB Entry DOI: 10.7270/Q2154MGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50520989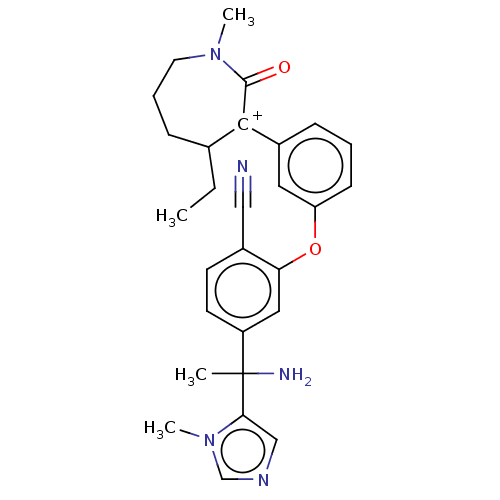 (CHEMBL4565280) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant FTase using [3H]FPP as substrate after 15 mins by scintillation counting analysis | Eur J Med Chem 162: 465-494 (2019) Article DOI: 10.1016/j.ejmech.2018.11.031 BindingDB Entry DOI: 10.7270/Q2154MGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM11823 (Azepane Derivative 4 | N-((3R,4R)-4-{[4-(2-fluoro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of PKBalpha (unknown origin) expressed in Sf9 insect cells using Biotin-SGRARTSSFAEPG as substrate after 30 mins by ELISA | Eur J Med Chem 162: 465-494 (2019) Article DOI: 10.1016/j.ejmech.2018.11.031 BindingDB Entry DOI: 10.7270/Q2154MGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252035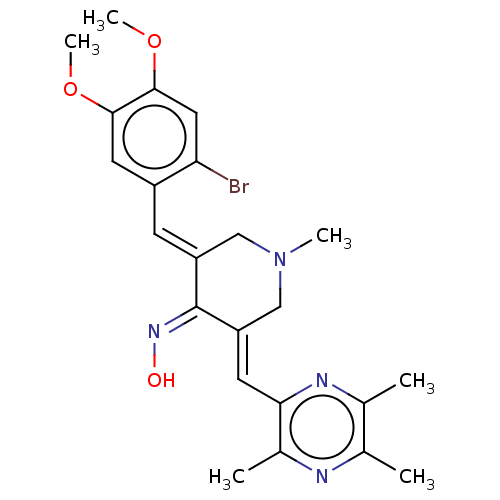 (CHEMBL4064361) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252033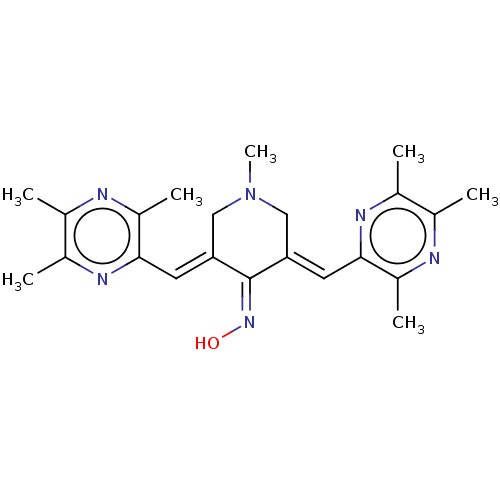 (CHEMBL4078023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161803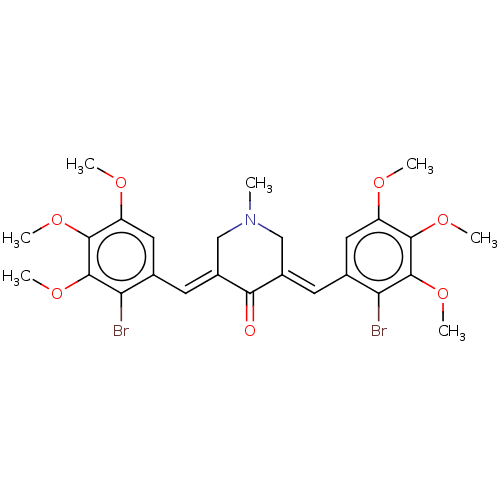 (CHEMBL3785375) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252089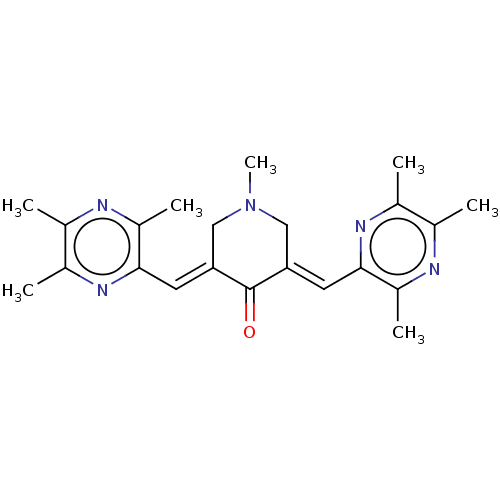 (CHEMBL4083642) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252090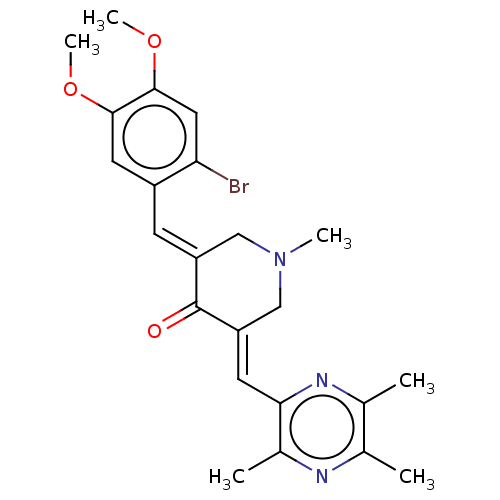 (CHEMBL4063890) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161797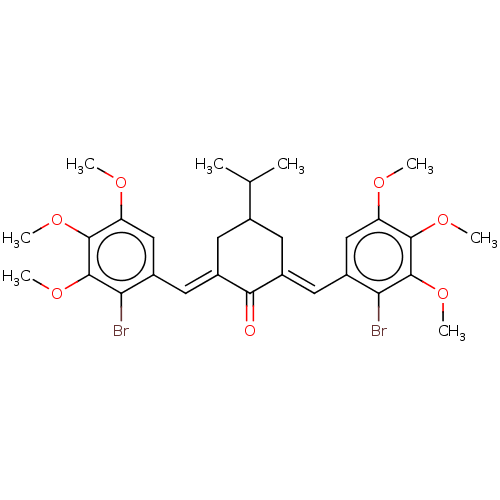 (CHEMBL3785402) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161802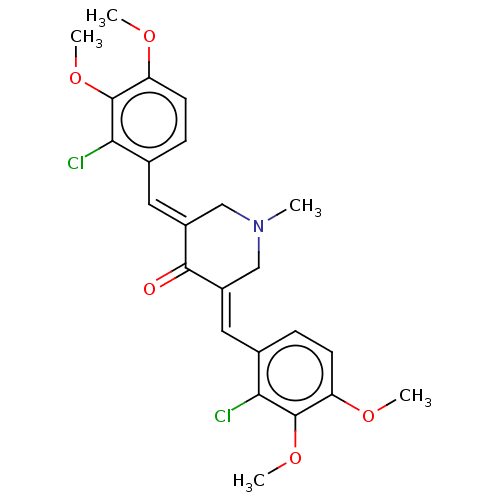 (CHEMBL3787299) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161796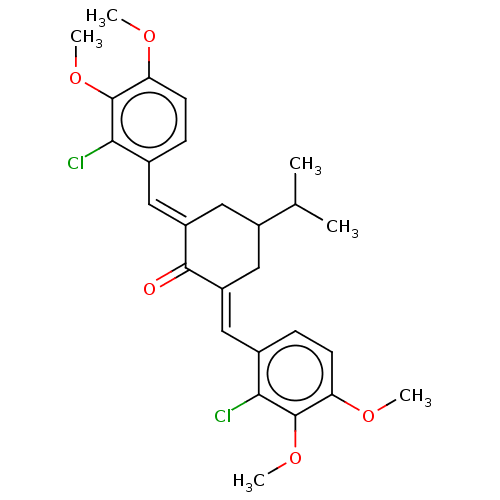 (CHEMBL3787250) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50252091 (CHEMBL4102284) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly preincubated with enzyme followed by GTP addition measured after 20 mins by spectrophotometric analysis | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50251945 (CHEMBL4100075) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50251946 (CHEMBL4104427) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly preincubated with enzyme followed by GTP addition measured after 20 mins by spectrophotometric analysis | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161801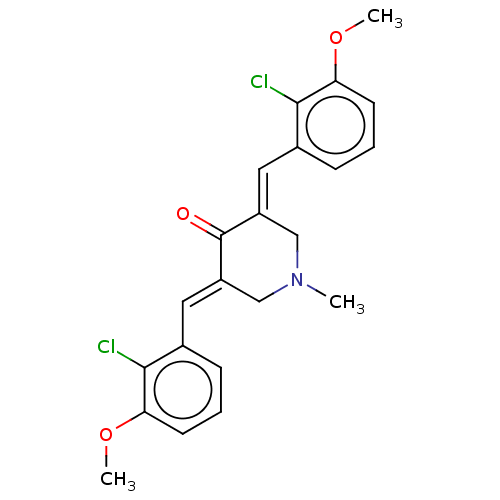 (CHEMBL3787510) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161795 (CHEMBL3787274) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161809 (CHEMBL3787119) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 111 total ) | Next | Last >> |