

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535693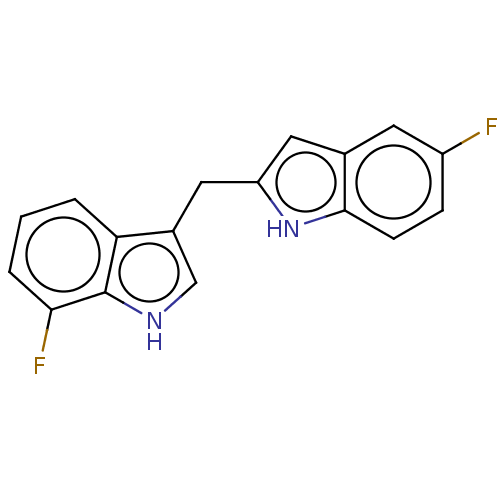 (CHEMBL4572783) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 24 hrs followed by medium replenishment and measured after 24 hrs by ELISA | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535683 (CHEMBL4549362) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535686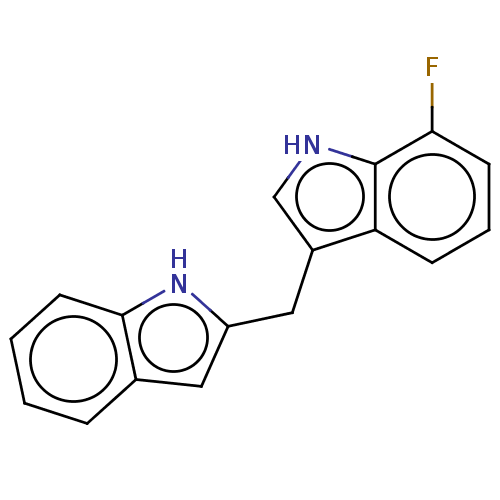 (CHEMBL4562557) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535688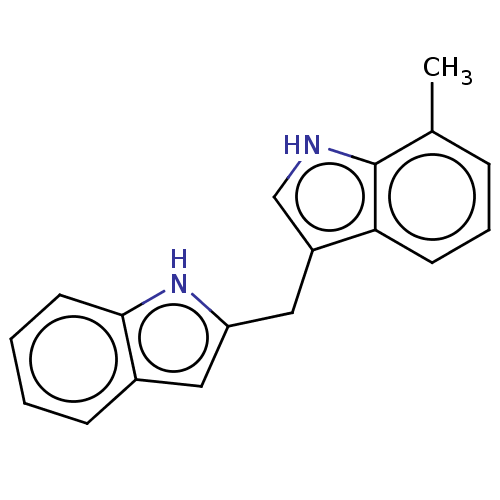 (CHEMBL4585306) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535690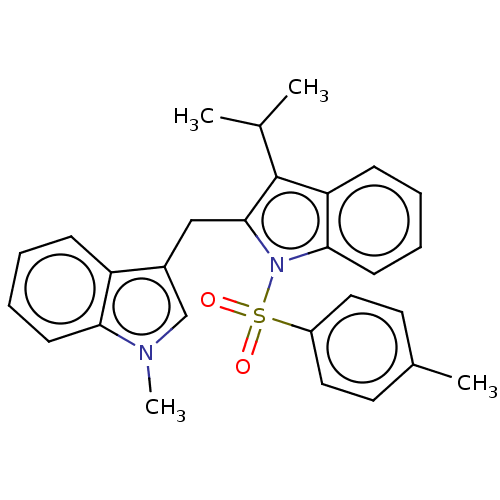 (CHEMBL4528279) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535687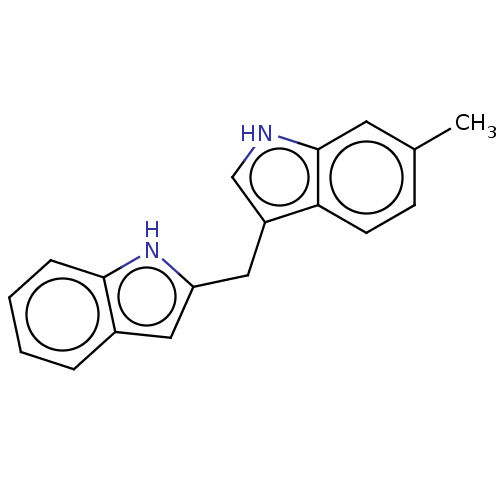 (CHEMBL4435394) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535689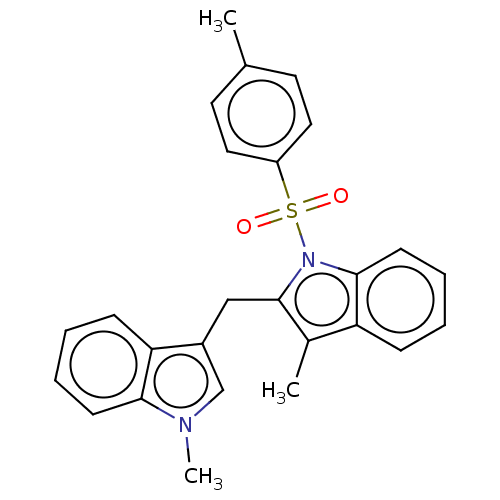 (CHEMBL4462933) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535685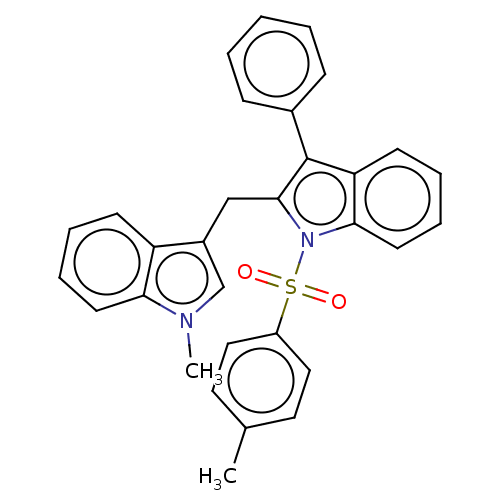 (CHEMBL4531399) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535691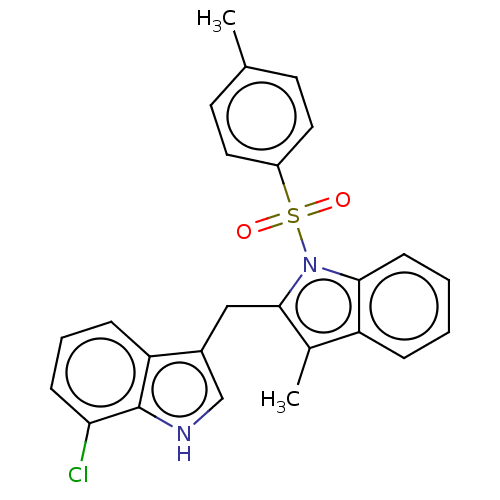 (CHEMBL4461546) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535692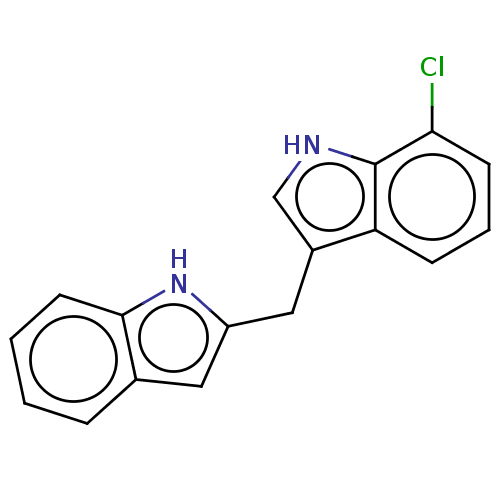 (CHEMBL4545753) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50535684 (CHEMBL4446521) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50049059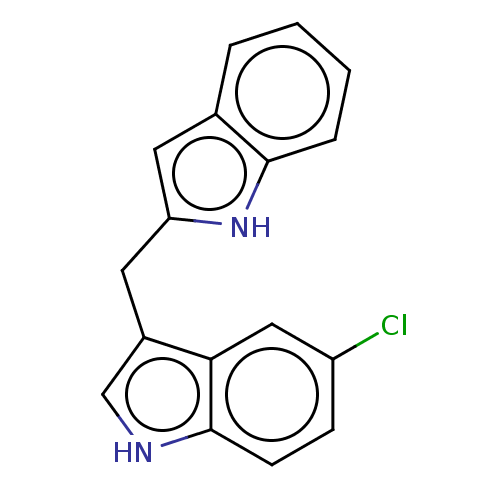 (CHEMBL3319503 | US10730833, Compound 10a | US99696...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 BindingDB Entry DOI: 10.7270/Q25142RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049062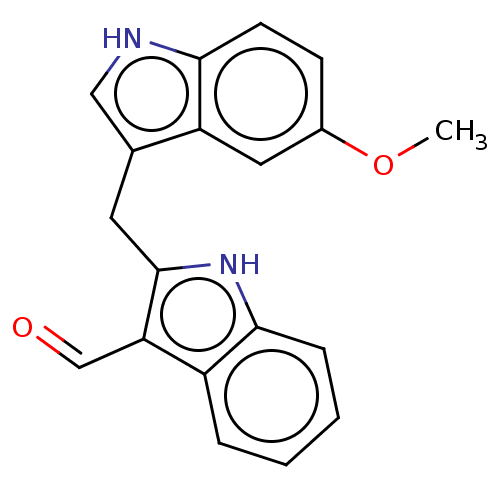 (CHEMBL3319500 | US10730833, Compound 11c | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049061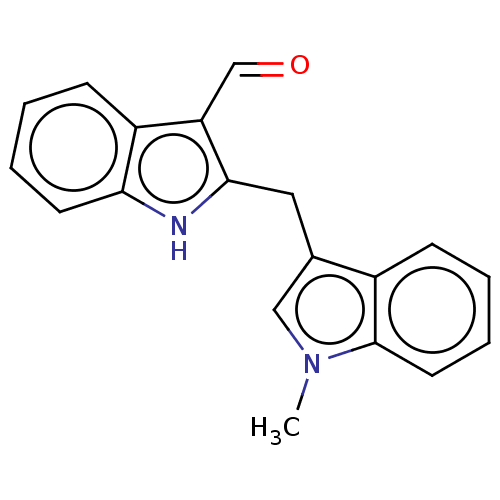 (CHEMBL3319501 | US10730833, Compound 11d | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049060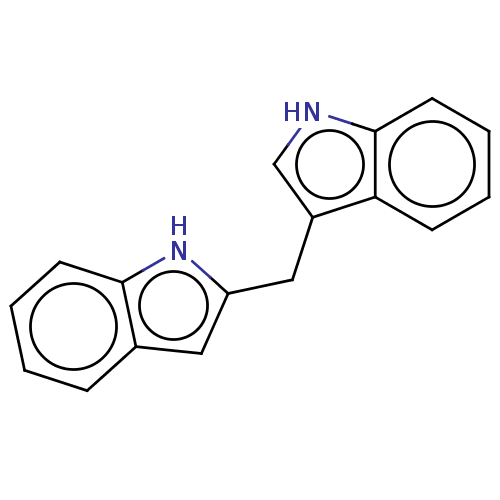 (CHEMBL3319502 | US10730833, Compound 6' | US996968...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 215 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049059 (CHEMBL3319503 | US10730833, Compound 10a | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | >6.70E+3 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049058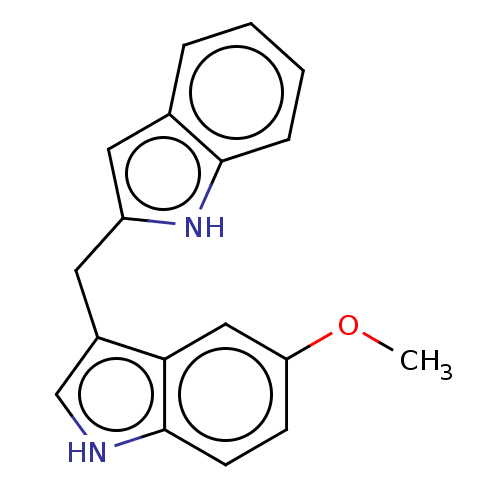 (CHEMBL3319504 | US10730833, Compound 10c | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049057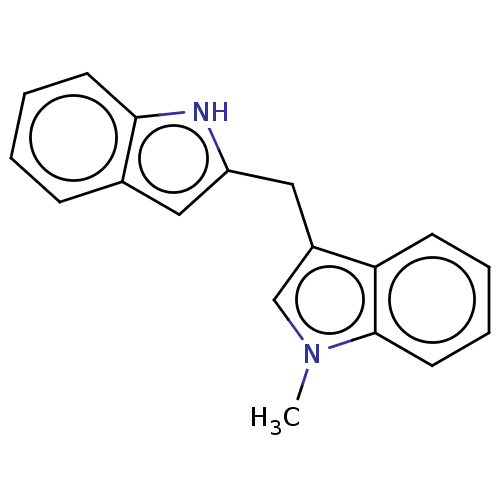 (CHEMBL3319505 | US10730833, Compound 10d | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049065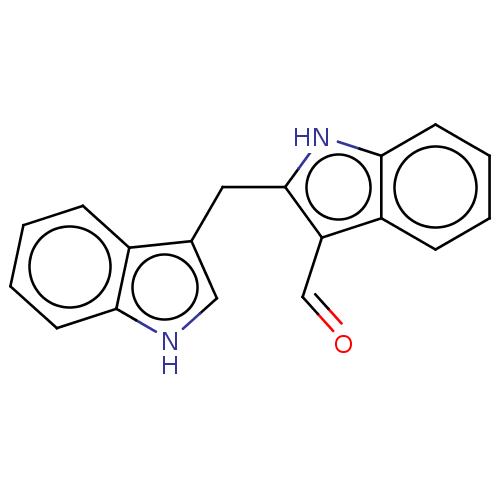 (CHEMBL3319497 | US10730833, Compound 6 | US9969686...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) BindingDB Entry DOI: 10.7270/Q2BK1GFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049064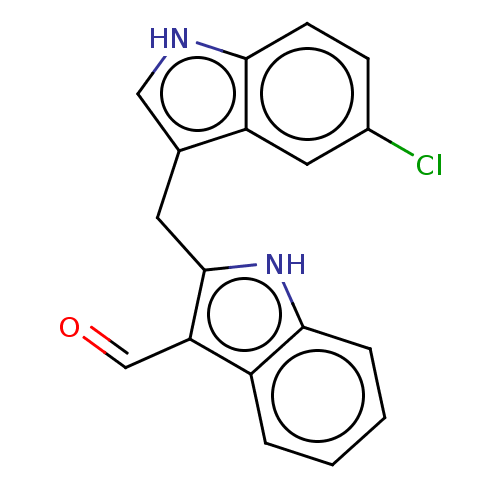 (CHEMBL3319498 | US10730833, Compound 11a | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) BindingDB Entry DOI: 10.7270/Q2BK1GFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049063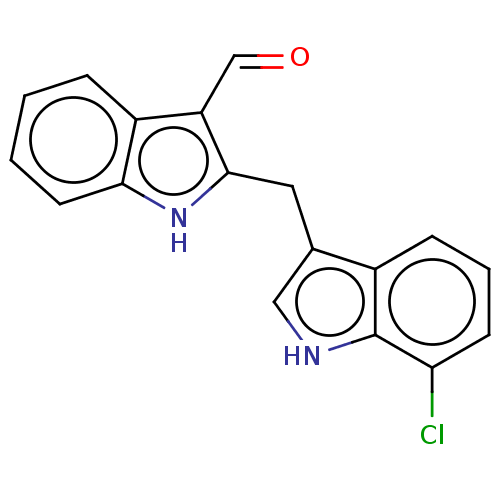 (CHEMBL3319499 | US10730833, Compound 11b | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) BindingDB Entry DOI: 10.7270/Q2BK1GFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049062 (CHEMBL3319500 | US10730833, Compound 11c | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) BindingDB Entry DOI: 10.7270/Q2BK1GFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049061 (CHEMBL3319501 | US10730833, Compound 11d | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) BindingDB Entry DOI: 10.7270/Q2BK1GFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049060 (CHEMBL3319502 | US10730833, Compound 6' | US996968...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 215 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) BindingDB Entry DOI: 10.7270/Q2BK1GFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049059 (CHEMBL3319503 | US10730833, Compound 10a | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | >6.70E+3 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) BindingDB Entry DOI: 10.7270/Q2BK1GFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049058 (CHEMBL3319504 | US10730833, Compound 10c | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) BindingDB Entry DOI: 10.7270/Q2BK1GFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049057 (CHEMBL3319505 | US10730833, Compound 10d | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) BindingDB Entry DOI: 10.7270/Q2BK1GFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50541261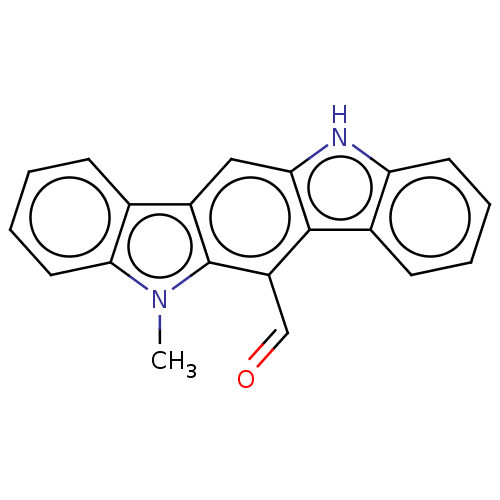 (CHEMBL4646273) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Agonist activity at AhR in human HepG2 cells assessed as induction of CYP1A1 expression after 24 hrs by ethoxyresorufin-O-deethylase assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126959 BindingDB Entry DOI: 10.7270/Q2WQ07B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50541262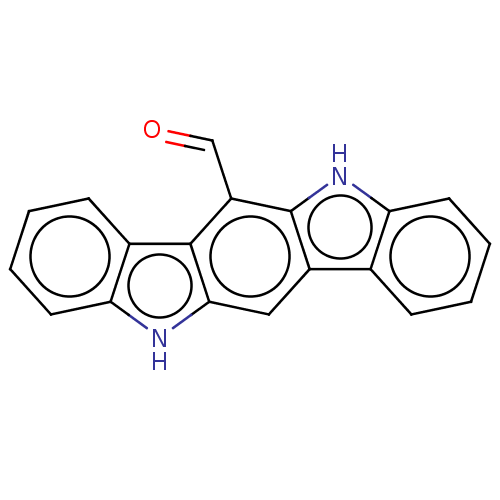 (6-Formylindolo[3,2-B]Carbazole | CHEMBL472031) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Agonist activity at AhR in human HepG2 cells assessed as induction of CYP1A1 expression after 24 hrs by ethoxyresorufin-O-deethylase assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126959 BindingDB Entry DOI: 10.7270/Q2WQ07B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565588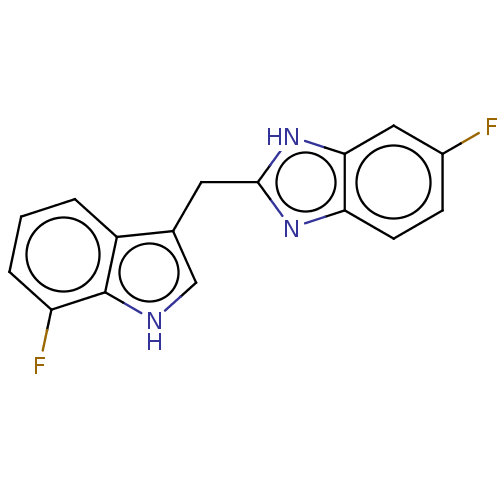 (CHEMBL4779430) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 348 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565589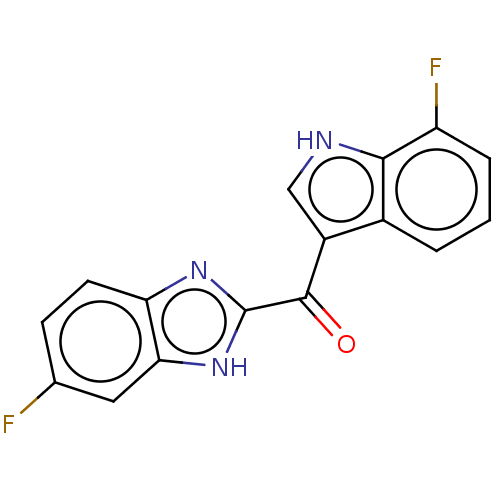 (CHEMBL4789986) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565590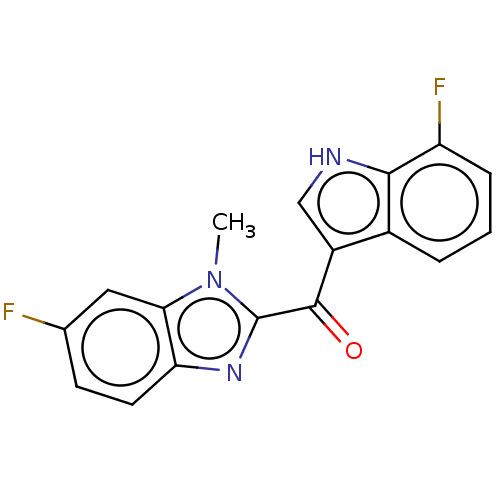 (CHEMBL4780905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565591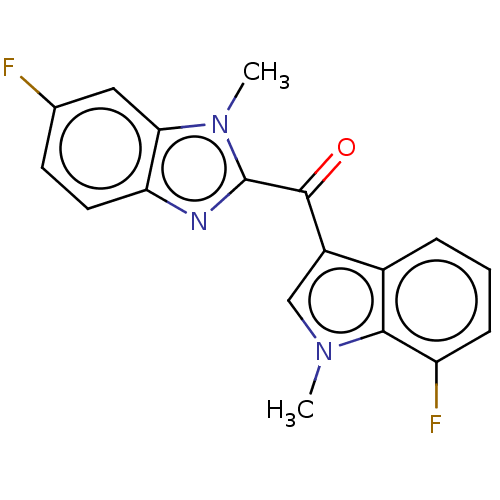 (CHEMBL4783308) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565592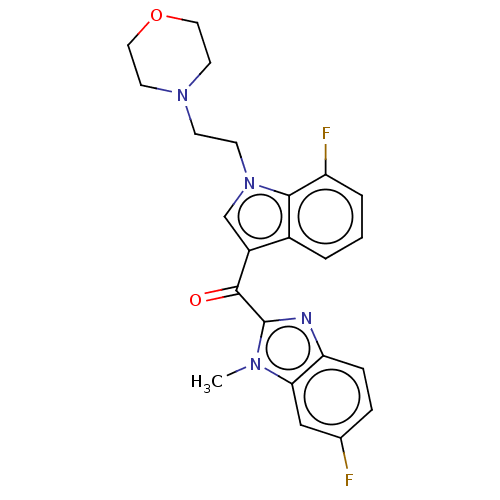 (CHEMBL4788701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565593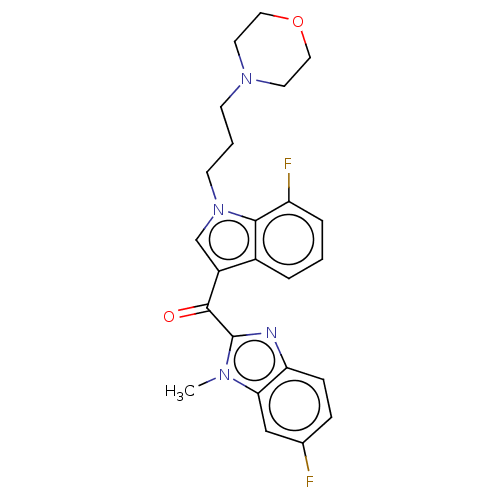 (CHEMBL4779800) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565594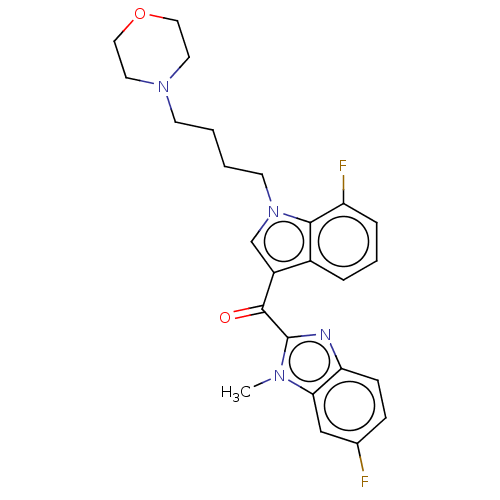 (CHEMBL4783828) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 88 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565595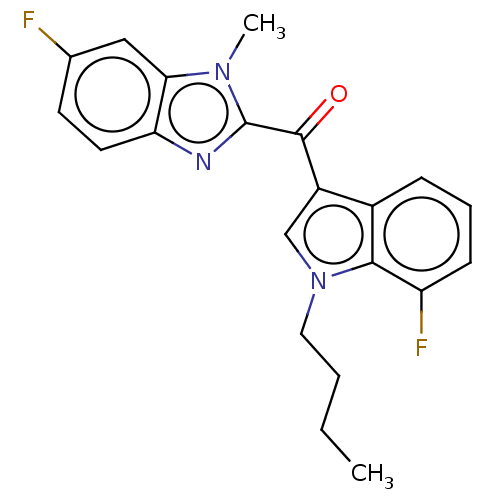 (CHEMBL4785006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565596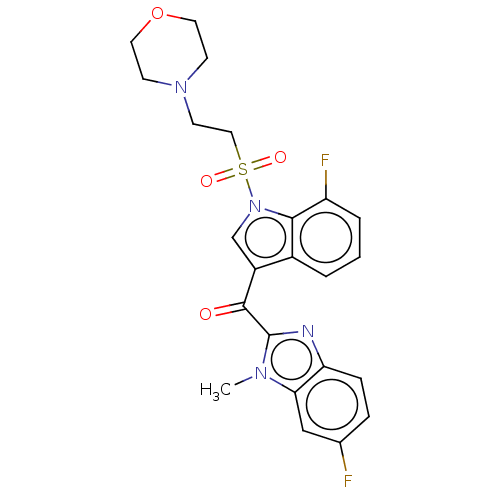 (CHEMBL4797870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049063 (CHEMBL3319499 | US10730833, Compound 11b | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049064 (CHEMBL3319498 | US10730833, Compound 11a | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049065 (CHEMBL3319497 | US10730833, Compound 6 | US9969686...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049065 (CHEMBL3319497 | US10730833, Compound 6 | US9969686...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assay | Bioorg Med Chem Lett 24: 4023-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.009 BindingDB Entry DOI: 10.7270/Q2TX3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049064 (CHEMBL3319498 | US10730833, Compound 11a | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assay | Bioorg Med Chem Lett 24: 4023-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.009 BindingDB Entry DOI: 10.7270/Q2TX3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049063 (CHEMBL3319499 | US10730833, Compound 11b | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assay | Bioorg Med Chem Lett 24: 4023-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.009 BindingDB Entry DOI: 10.7270/Q2TX3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049062 (CHEMBL3319500 | US10730833, Compound 11c | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assay | Bioorg Med Chem Lett 24: 4023-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.009 BindingDB Entry DOI: 10.7270/Q2TX3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049061 (CHEMBL3319501 | US10730833, Compound 11d | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assay | Bioorg Med Chem Lett 24: 4023-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.009 BindingDB Entry DOI: 10.7270/Q2TX3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049060 (CHEMBL3319502 | US10730833, Compound 6' | US996968...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 215 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assay | Bioorg Med Chem Lett 24: 4023-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.009 BindingDB Entry DOI: 10.7270/Q2TX3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049059 (CHEMBL3319503 | US10730833, Compound 10a | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >6.70E+3 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assay | Bioorg Med Chem Lett 24: 4023-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.009 BindingDB Entry DOI: 10.7270/Q2TX3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50565597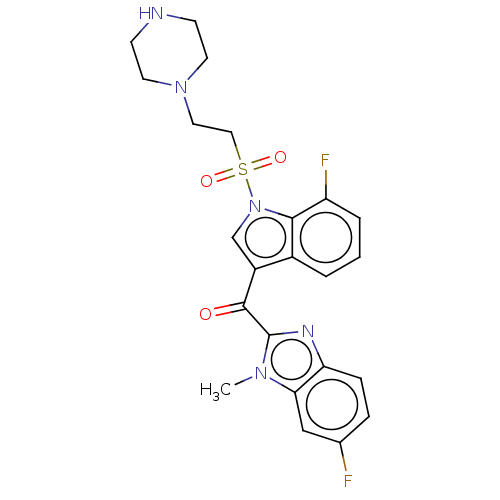 (CHEMBL4783880) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 161 | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of PCSK9 in human HepG2 cells assessed as reduction in PCSK9 expression incubated for 24 hrs prior to compound washout followed by compoun... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112678 BindingDB Entry DOI: 10.7270/Q2F193GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049057 (CHEMBL3319505 | US10730833, Compound 10d | US99696...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assay | Bioorg Med Chem Lett 24: 4023-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.009 BindingDB Entry DOI: 10.7270/Q2TX3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |