 Found 110 hits with Last Name = 'rouen' and Initial = 'gp'
Found 110 hits with Last Name = 'rouen' and Initial = 'gp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167799
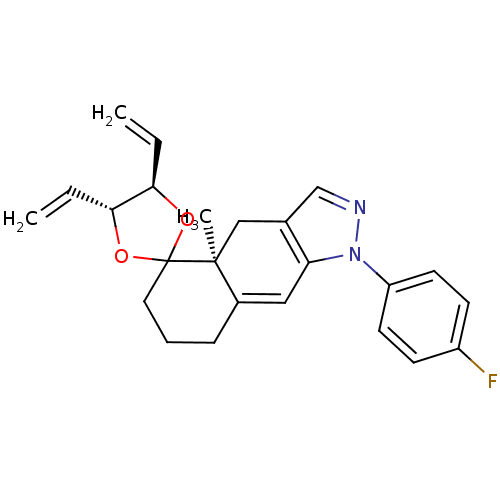
(1-(4-fluorophenyl)-4a-methyl-4',5'-divinyl-(4'R,4a...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCCC21O[C@H](C=C)[C@H](O1)C=C)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C24H25FN2O2/c1-4-21-22(5-2)29-24(28-21)12-6-7-17-13-20-16(14-23(17,24)3)15-26-27(20)19-10-8-18(25)9-11-19/h4-5,8-11,13,15,21-22H,1-2,6-7,12,14H2,3H3/t21-,22-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human glucocorticoid receptor |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167799

(1-(4-fluorophenyl)-4a-methyl-4',5'-divinyl-(4'R,4a...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCCC21O[C@H](C=C)[C@H](O1)C=C)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C24H25FN2O2/c1-4-21-22(5-2)29-24(28-21)12-6-7-17-13-20-16(14-23(17,24)3)15-26-27(20)19-10-8-18(25)9-11-19/h4-5,8-11,13,15,21-22H,1-2,6-7,12,14H2,3H3/t21-,22-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167810
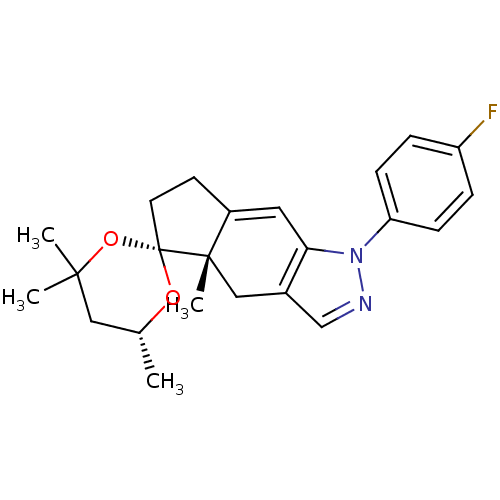
(1-(4-fluorophenyl)-4',4',4a,6'-tetramethyl-(4aS,6'...)Show SMILES C[C@@H]1CC(C)(C)O[C@@]2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O1 |t:10| Show InChI InChI=1S/C23H27FN2O2/c1-15-12-21(2,3)28-23(27-15)10-9-17-11-20-16(13-22(17,23)4)14-25-26(20)19-7-5-18(24)6-8-19/h5-8,11,14-15H,9-10,12-13H2,1-4H3/t15-,22+,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167815
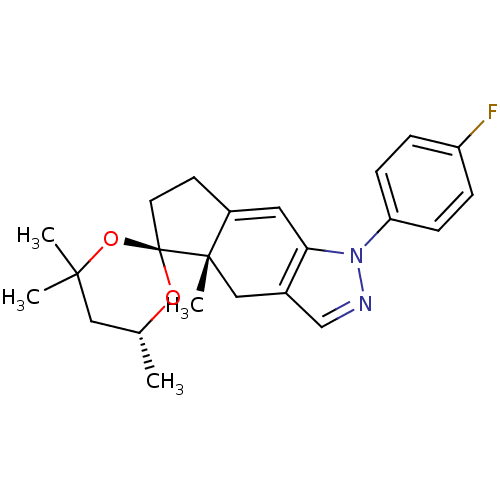
(1-(4-fluorophenyl)-4',4',4a,6'-tetramethyl-(4aS,6'...)Show SMILES C[C@@H]1CC(C)(C)O[C@]2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O1 |t:10| Show InChI InChI=1S/C23H27FN2O2/c1-15-12-21(2,3)28-23(27-15)10-9-17-11-20-16(13-22(17,23)4)14-25-26(20)19-7-5-18(24)6-8-19/h5-8,11,14-15H,9-10,12-13H2,1-4H3/t15-,22+,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167811
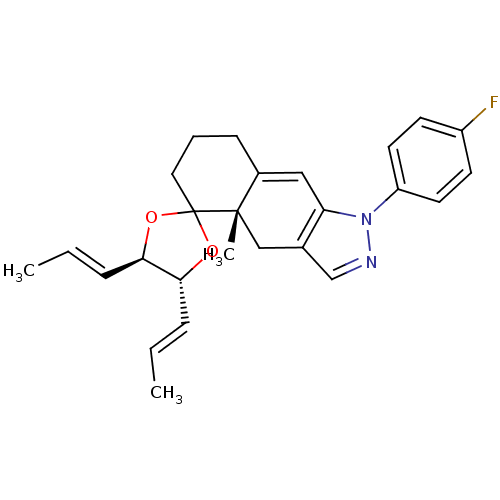
(1-(4-fluorophenyl)-4a-methyl-4',5'-di[(E)-1-propen...)Show SMILES C\C=C\[C@H]1OC2(CCCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1\C=C\C |t:9| Show InChI InChI=1S/C26H29FN2O2/c1-4-7-23-24(8-5-2)31-26(30-23)14-6-9-19-15-22-18(16-25(19,26)3)17-28-29(22)21-12-10-20(27)11-13-21/h4-5,7-8,10-13,15,17,23-24H,6,9,14,16H2,1-3H3/b7-4+,8-5+/t23-,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167809
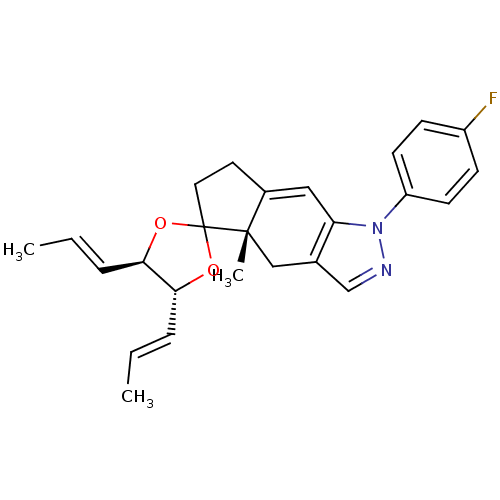
(1-(4-fluorophenyl)-4a-methyl-4',5'-di[(E)-1-propen...)Show SMILES C\C=C\[C@H]1OC2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1\C=C\C |t:8| Show InChI InChI=1S/C25H27FN2O2/c1-4-6-22-23(7-5-2)30-25(29-22)13-12-18-14-21-17(15-24(18,25)3)16-27-28(21)20-10-8-19(26)9-11-20/h4-11,14,16,22-23H,12-13,15H2,1-3H3/b6-4+,7-5+/t22-,23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167792
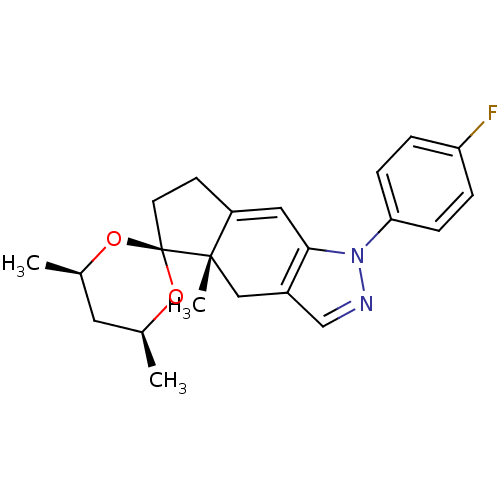
(1-(4-fluorophenyl)-4',4a,6'-trimethyl-(4'R,4aS,6'S...)Show SMILES C[C@H]1C[C@@H](C)O[C@]2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O1 |t:9| Show InChI InChI=1S/C22H25FN2O2/c1-14-10-15(2)27-22(26-14)9-8-17-11-20-16(12-21(17,22)3)13-24-25(20)19-6-4-18(23)5-7-19/h4-7,11,13-15H,8-10,12H2,1-3H3/t14-,15+,21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167797
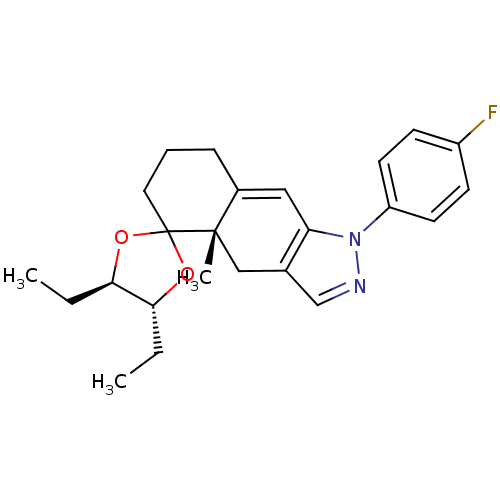
(4',5'-diethyl-1-(4-fluorophenyl)-4a-methyl-(4'R,4a...)Show SMILES CC[C@H]1OC2(CCCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1CC |t:8| Show InChI InChI=1S/C24H29FN2O2/c1-4-21-22(5-2)29-24(28-21)12-6-7-17-13-20-16(14-23(17,24)3)15-26-27(20)19-10-8-18(25)9-11-19/h8-11,13,15,21-22H,4-7,12,14H2,1-3H3/t21-,22-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167807
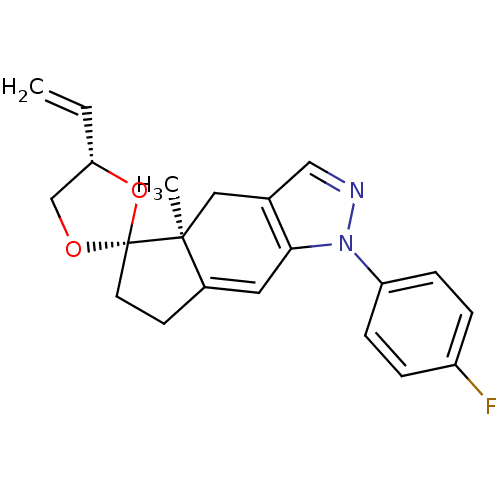
(1-(4-fluorophenyl)-4a-methyl-4'-vinyl-(4'S,4aS,5'R...)Show SMILES C[C@]12Cc3cnn(c3C=C1CC[C@]21OC[C@@H](O1)C=C)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C21H21FN2O2/c1-3-18-13-25-21(26-18)9-8-15-10-19-14(11-20(15,21)2)12-23-24(19)17-6-4-16(22)5-7-17/h3-7,10,12,18H,1,8-9,11,13H2,2H3/t18-,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167798
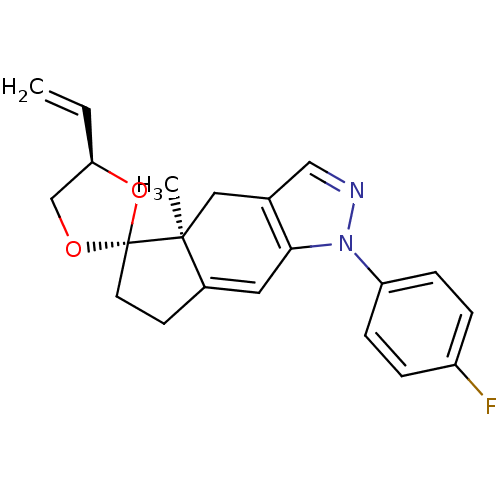
(1-(4-fluorophenyl)-4a-methyl-4'-vinyl-(4'R,4aS,5'R...)Show SMILES C[C@]12Cc3cnn(c3C=C1CC[C@]21OC[C@H](O1)C=C)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C21H21FN2O2/c1-3-18-13-25-21(26-18)9-8-15-10-19-14(11-20(15,21)2)12-23-24(19)17-6-4-16(22)5-7-17/h3-7,10,12,18H,1,8-9,11,13H2,2H3/t18-,20+,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167806
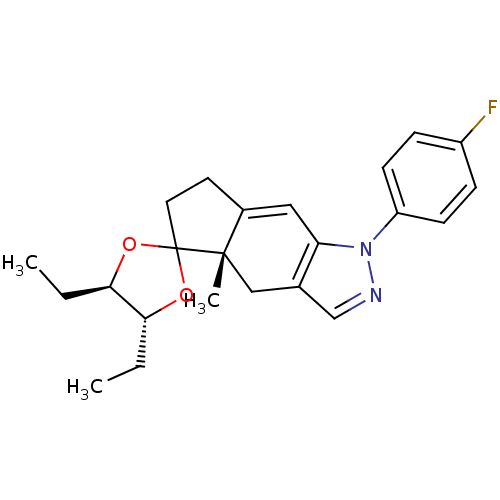
(4',5'-diethyl-1-(4-fluorophenyl)-4a-methyl-(4'R,4a...)Show SMILES CC[C@H]1OC2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1CC |t:7| Show InChI InChI=1S/C23H27FN2O2/c1-4-20-21(5-2)28-23(27-20)11-10-16-12-19-15(13-22(16,23)3)14-25-26(19)18-8-6-17(24)7-9-18/h6-9,12,14,20-21H,4-5,10-11,13H2,1-3H3/t20-,21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167790
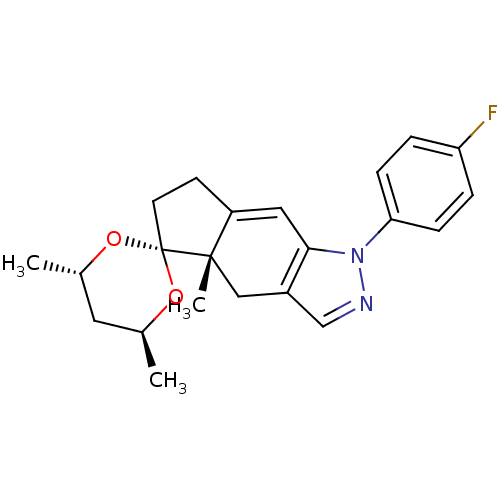
(1-(4-fluorophenyl)-4',4a,6'-trimethyl-(4'S,4aS,6'S...)Show SMILES C[C@H]1C[C@H](C)O[C@@]2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O1 |t:9| Show InChI InChI=1S/C22H25FN2O2/c1-14-10-15(2)27-22(26-14)9-8-17-11-20-16(12-21(17,22)3)13-24-25(20)19-6-4-18(23)5-7-19/h4-7,11,13-15H,8-10,12H2,1-3H3/t14-,15-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human glucocorticoid receptor |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167790

(1-(4-fluorophenyl)-4',4a,6'-trimethyl-(4'S,4aS,6'S...)Show SMILES C[C@H]1C[C@H](C)O[C@@]2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O1 |t:9| Show InChI InChI=1S/C22H25FN2O2/c1-14-10-15(2)27-22(26-14)9-8-17-11-20-16(12-21(17,22)3)13-24-25(20)19-6-4-18(23)5-7-19/h4-7,11,13-15H,8-10,12H2,1-3H3/t14-,15-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167796
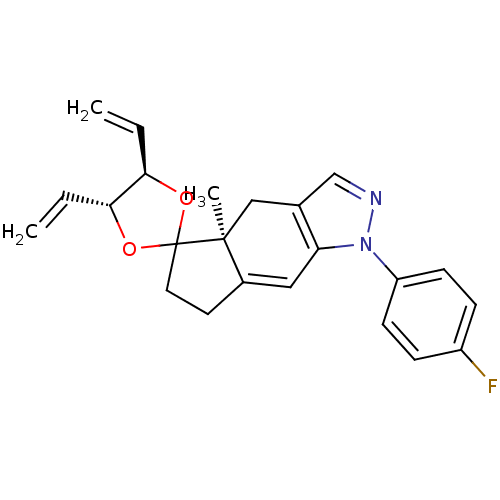
(1-(4-fluorophenyl)-4a-methyl-4',5'-divinyl-(4'R,4a...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCC21O[C@H](C=C)[C@H](O1)C=C)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C23H23FN2O2/c1-4-20-21(5-2)28-23(27-20)11-10-16-12-19-15(13-22(16,23)3)14-25-26(19)18-8-6-17(24)7-9-18/h4-9,12,14,20-21H,1-2,10-11,13H2,3H3/t20-,21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human glucocorticoid receptor |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167796

(1-(4-fluorophenyl)-4a-methyl-4',5'-divinyl-(4'R,4a...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCC21O[C@H](C=C)[C@H](O1)C=C)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C23H23FN2O2/c1-4-20-21(5-2)28-23(27-20)11-10-16-12-19-15(13-22(16,23)3)14-25-26(19)18-8-6-17(24)7-9-18/h4-9,12,14,20-21H,1-2,10-11,13H2,3H3/t20-,21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167793
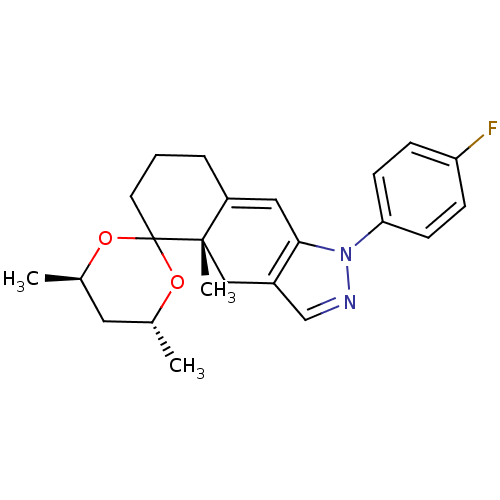
(1-(4-fluorophenyl)-4',4a,6'-trimethyl-(4'R,4aS,6'R...)Show SMILES C[C@@H]1C[C@@H](C)OC2(CCCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O1 |t:10| Show InChI InChI=1S/C23H27FN2O2/c1-15-11-16(2)28-23(27-15)10-4-5-18-12-21-17(13-22(18,23)3)14-25-26(21)20-8-6-19(24)7-9-20/h6-9,12,14-16H,4-5,10-11,13H2,1-3H3/t15-,16-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167804
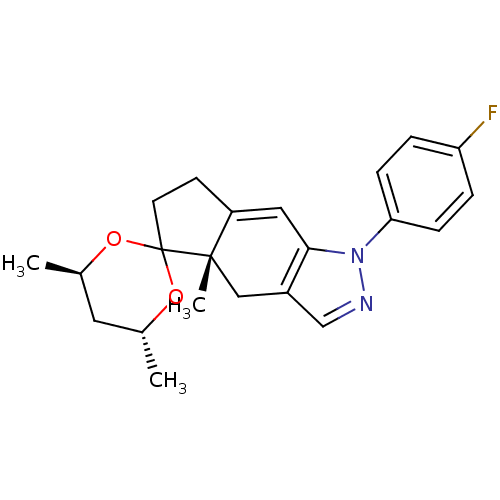
(1-(4-fluorophenyl)-4',4a,6'-trimethyl-(4'R,4aS,6'R...)Show SMILES C[C@@H]1C[C@@H](C)OC2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O1 |t:9| Show InChI InChI=1S/C22H25FN2O2/c1-14-10-15(2)27-22(26-14)9-8-17-11-20-16(12-21(17,22)3)13-24-25(20)19-6-4-18(23)5-7-19/h4-7,11,13-15H,8-10,12H2,1-3H3/t14-,15-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167812
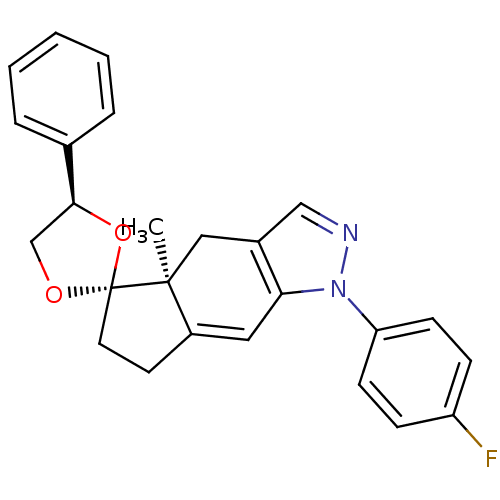
(1-(4-fluorophenyl)-4a-methyl-4'-phenyl-(4'R,4aS,5'...)Show SMILES C[C@]12Cc3cnn(c3C=C1CC[C@]21OC[C@H](O1)c1ccccc1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C25H23FN2O2/c1-24-14-18-15-27-28(21-9-7-20(26)8-10-21)22(18)13-19(24)11-12-25(24)29-16-23(30-25)17-5-3-2-4-6-17/h2-10,13,15,23H,11-12,14,16H2,1H3/t23-,24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167789
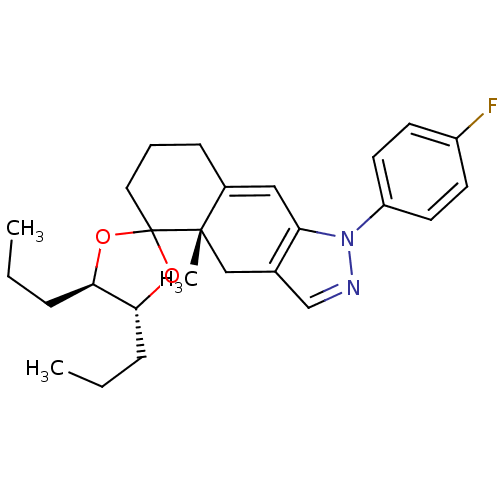
(1-(4-fluorophenyl)-4a-methyl-4',5'-dipropyl-(4'R,4...)Show SMILES CCC[C@H]1OC2(CCCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1CCC |t:9| Show InChI InChI=1S/C26H33FN2O2/c1-4-7-23-24(8-5-2)31-26(30-23)14-6-9-19-15-22-18(16-25(19,26)3)17-28-29(22)21-12-10-20(27)11-13-21/h10-13,15,17,23-24H,4-9,14,16H2,1-3H3/t23-,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167788
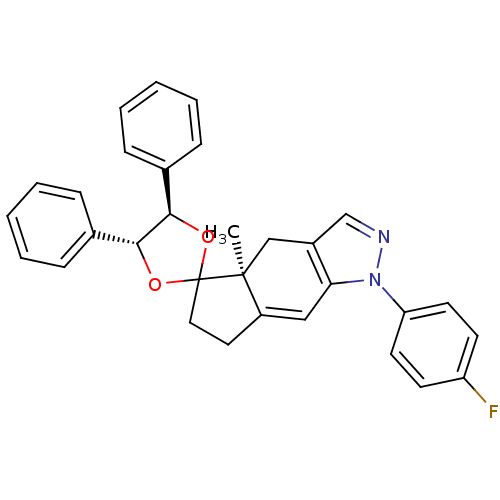
(1-(4-fluorophenyl)-4a-methyl-4',5'-diphenyl-(4'R,4...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCC21O[C@@H]([C@H](O1)c1ccccc1)c1ccccc1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C31H27FN2O2/c1-30-19-23-20-33-34(26-14-12-25(32)13-15-26)27(23)18-24(30)16-17-31(30)35-28(21-8-4-2-5-9-21)29(36-31)22-10-6-3-7-11-22/h2-15,18,20,28-29H,16-17,19H2,1H3/t28-,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167817
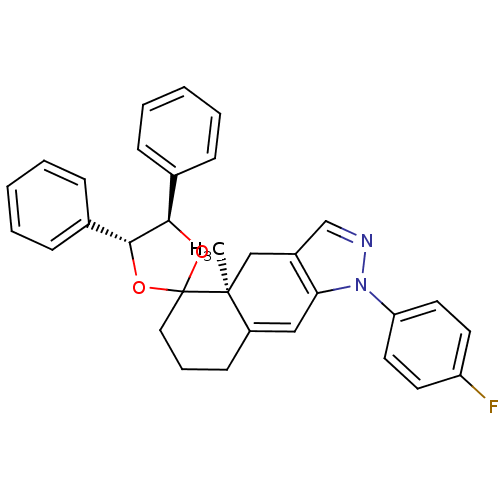
(1-(4-fluorophenyl)-4a-methyl-4',5'-diphenyl-(4'R,4...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCCC21O[C@@H]([C@H](O1)c1ccccc1)c1ccccc1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C32H29FN2O2/c1-31-20-24-21-34-35(27-16-14-26(33)15-17-27)28(24)19-25(31)13-8-18-32(31)36-29(22-9-4-2-5-10-22)30(37-32)23-11-6-3-7-12-23/h2-7,9-12,14-17,19,21,29-30H,8,13,18,20H2,1H3/t29-,30-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167818
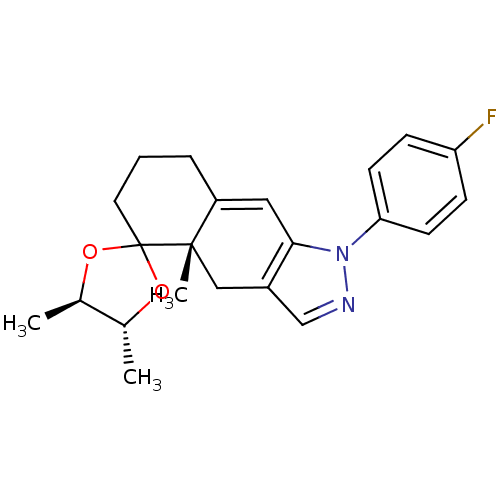
(1-(4-fluorophenyl)-4',4a,5'-trimethyl-(4'R,4aS,5'R...)Show SMILES C[C@H]1OC2(CCCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1C |t:7| Show InChI InChI=1S/C22H25FN2O2/c1-14-15(2)27-22(26-14)10-4-5-17-11-20-16(12-21(17,22)3)13-24-25(20)19-8-6-18(23)7-9-19/h6-9,11,13-15H,4-5,10,12H2,1-3H3/t14-,15-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167819
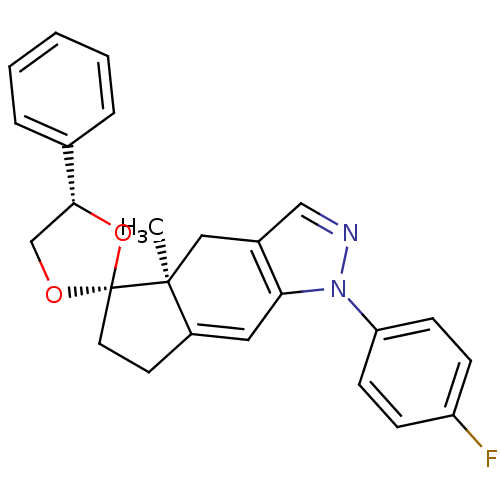
(1-(4-fluorophenyl)-4a-methyl-4'-phenyl-(4'S,4aS,5'...)Show SMILES C[C@]12Cc3cnn(c3C=C1CC[C@]21OC[C@@H](O1)c1ccccc1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C25H23FN2O2/c1-24-14-18-15-27-28(21-9-7-20(26)8-10-21)22(18)13-19(24)11-12-25(24)29-16-23(30-25)17-5-3-2-4-6-17/h2-10,13,15,23H,11-12,14,16H2,1H3/t23-,24+,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167794
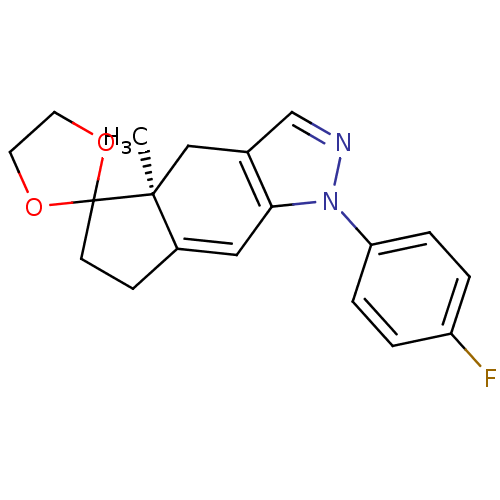
(1-(4-fluorophenyl)-4a-methyl-(4aS)-spiro[1,4,4a,5,...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCC21OCCO1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C19H19FN2O2/c1-18-11-13-12-21-22(16-4-2-15(20)3-5-16)17(13)10-14(18)6-7-19(18)23-8-9-24-19/h2-5,10,12H,6-9,11H2,1H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167794

(1-(4-fluorophenyl)-4a-methyl-(4aS)-spiro[1,4,4a,5,...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCC21OCCO1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C19H19FN2O2/c1-18-11-13-12-21-22(16-4-2-15(20)3-5-16)17(13)10-14(18)6-7-19(18)23-8-9-24-19/h2-5,10,12H,6-9,11H2,1H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167808

(1-(4-fluorophenyl)-4a-methyl-(4aS)-spiro[4,4a,5,6,...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCCC21OCCO1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C20H21FN2O2/c1-19-12-14-13-22-23(17-6-4-16(21)5-7-17)18(14)11-15(19)3-2-8-20(19)24-9-10-25-20/h4-7,11,13H,2-3,8-10,12H2,1H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167808

(1-(4-fluorophenyl)-4a-methyl-(4aS)-spiro[4,4a,5,6,...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCCC21OCCO1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C20H21FN2O2/c1-19-12-14-13-22-23(17-6-4-16(21)5-7-17)18(14)11-15(19)3-2-8-20(19)24-9-10-25-20/h4-7,11,13H,2-3,8-10,12H2,1H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human glucocorticoid receptor |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167816
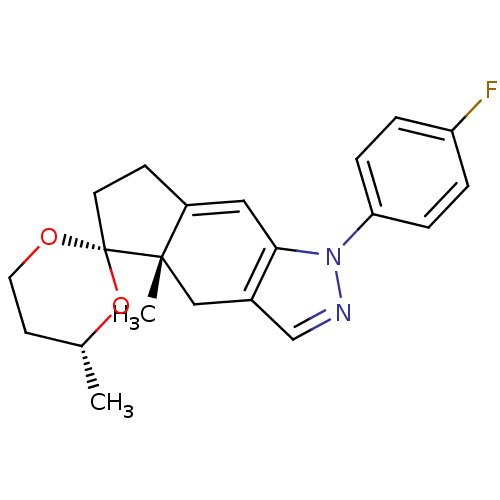
(1-(4-fluorophenyl)-4',4a-dimethyl-(4'R,4aS,5'S)-sp...)Show SMILES C[C@@H]1CCO[C@@]2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O1 |t:8| Show InChI InChI=1S/C21H23FN2O2/c1-14-8-10-25-21(26-14)9-7-16-11-19-15(12-20(16,21)2)13-23-24(19)18-5-3-17(22)4-6-18/h3-6,11,13-14H,7-10,12H2,1-2H3/t14-,20+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167800
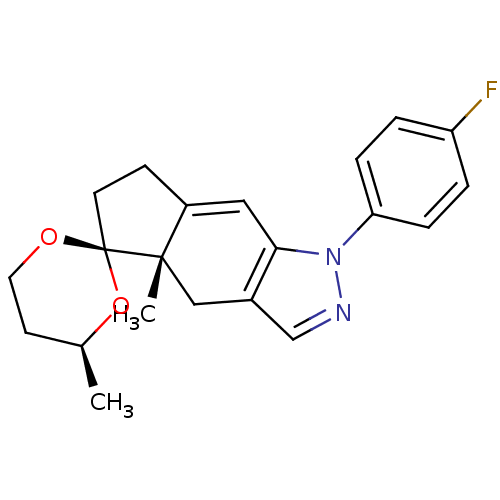
(1-(4-fluorophenyl)-4',4a-dimethyl-(4'S,4aS,5'R)-sp...)Show SMILES C[C@H]1CCO[C@]2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O1 |t:8| Show InChI InChI=1S/C21H23FN2O2/c1-14-8-10-25-21(26-14)9-7-16-11-19-15(12-20(16,21)2)13-23-24(19)18-5-3-17(22)4-6-18/h3-6,11,13-14H,7-10,12H2,1-2H3/t14-,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167805
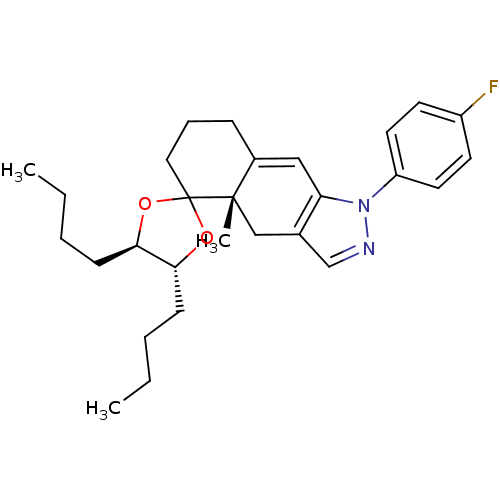
(4',5'-dibutyl-1-(4-fluorophenyl)-4a-methyl-(4'R,4a...)Show SMILES CCCC[C@H]1OC2(CCCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1CCCC |t:10| Show InChI InChI=1S/C28H37FN2O2/c1-4-6-10-25-26(11-7-5-2)33-28(32-25)16-8-9-21-17-24-20(18-27(21,28)3)19-30-31(24)23-14-12-22(29)13-15-23/h12-15,17,19,25-26H,4-11,16,18H2,1-3H3/t25-,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167795

(1-(4-fluorophenyl)-4',4a,5'-trimethyl-(4'R,4aS,5'R...)Show SMILES C[C@H]1OC2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1C |t:6| Show InChI InChI=1S/C21H23FN2O2/c1-13-14(2)26-21(25-13)9-8-16-10-19-15(11-20(16,21)3)12-23-24(19)18-6-4-17(22)5-7-18/h4-7,10,12-14H,8-9,11H2,1-3H3/t13-,14-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human glucocorticoid receptor |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167795

(1-(4-fluorophenyl)-4',4a,5'-trimethyl-(4'R,4aS,5'R...)Show SMILES C[C@H]1OC2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1C |t:6| Show InChI InChI=1S/C21H23FN2O2/c1-13-14(2)26-21(25-13)9-8-16-10-19-15(11-20(16,21)3)12-23-24(19)18-6-4-17(22)5-7-18/h4-7,10,12-14H,8-9,11H2,1-3H3/t13-,14-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167802
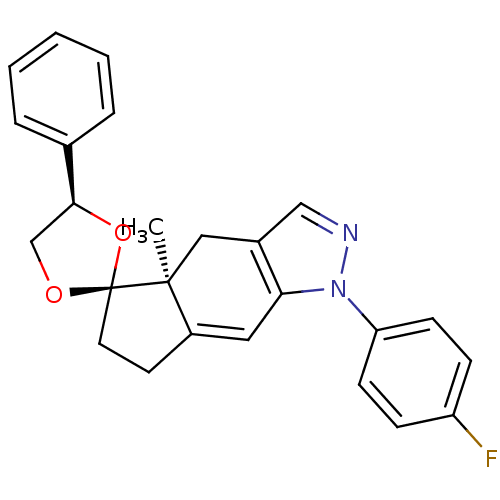
(1-(4-fluorophenyl)-4a-methyl-4'-phenyl-(4'R,4aS,5'...)Show SMILES C[C@]12Cc3cnn(c3C=C1CC[C@@]21OC[C@H](O1)c1ccccc1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C25H23FN2O2/c1-24-14-18-15-27-28(21-9-7-20(26)8-10-21)22(18)13-19(24)11-12-25(24)29-16-23(30-25)17-5-3-2-4-6-17/h2-10,13,15,23H,11-12,14,16H2,1H3/t23-,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167787
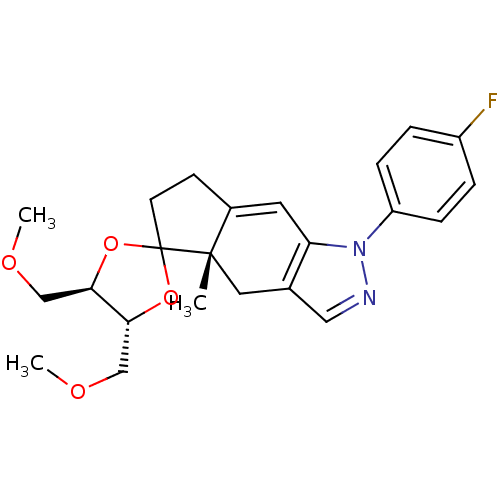
(1-(4-fluorophenyl)-4',5'-di(methoxymethyl)-4a-meth...)Show SMILES COC[C@H]1OC2(CCC3=Cc4c(C[C@]23C)cnn4-c2ccc(F)cc2)O[C@@H]1COC |t:8| Show InChI InChI=1S/C23H27FN2O4/c1-22-11-15-12-25-26(18-6-4-17(24)5-7-18)19(15)10-16(22)8-9-23(22)29-20(13-27-2)21(30-23)14-28-3/h4-7,10,12,20-21H,8-9,11,13-14H2,1-3H3/t20-,21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167803
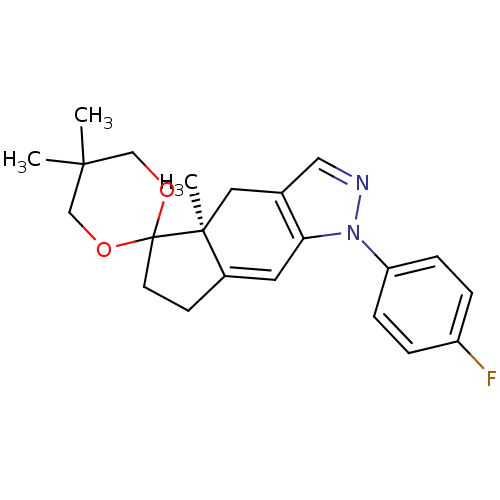
(1-(4-fluorophenyl)-4a,5',5'-trimethyl-(4aS)-spiro[...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCC21OCC(C)(C)CO1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C22H25FN2O2/c1-20(2)13-26-22(27-14-20)9-8-16-10-19-15(11-21(16,22)3)12-24-25(19)18-6-4-17(23)5-7-18/h4-7,10,12H,8-9,11,13-14H2,1-3H3/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167813
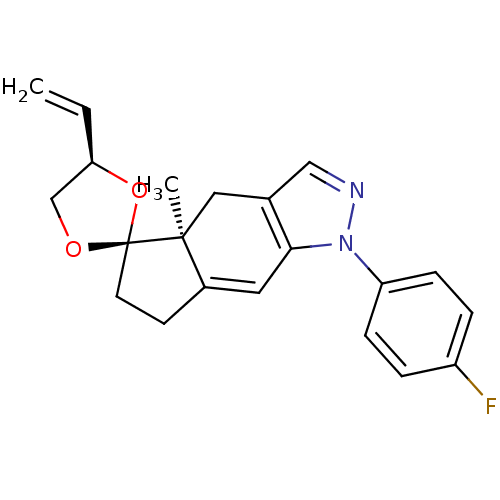
(1-(4-fluorophenyl)-4a-methyl-4'-vinyl-(4'R,4aS,5'S...)Show SMILES C[C@]12Cc3cnn(c3C=C1CC[C@@]21OC[C@H](O1)C=C)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C21H21FN2O2/c1-3-18-13-25-21(26-18)9-8-15-10-19-14(11-20(15,21)2)12-23-24(19)17-6-4-16(22)5-7-17/h3-7,10,12,18H,1,8-9,11,13H2,2H3/t18-,20+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167814
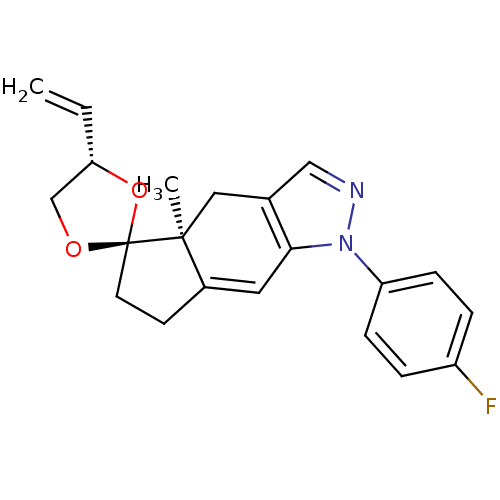
(1-(4-fluorophenyl)-4a-methyl-4'-vinyl-(4'S,4aS,5'S...)Show SMILES C[C@]12Cc3cnn(c3C=C1CC[C@@]21OC[C@@H](O1)C=C)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C21H21FN2O2/c1-3-18-13-25-21(26-18)9-8-15-10-19-14(11-20(15,21)2)12-23-24(19)17-6-4-16(22)5-7-17/h3-7,10,12,18H,1,8-9,11,13H2,2H3/t18-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167791

(1-(4-fluorophenyl)-4a-methyl-4'-phenyl-(4'S,4aS,5'...)Show SMILES C[C@]12Cc3cnn(c3C=C1CC[C@@]21OC[C@@H](O1)c1ccccc1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C25H23FN2O2/c1-24-14-18-15-27-28(21-9-7-20(26)8-10-21)22(18)13-19(24)11-12-25(24)29-16-23(30-25)17-5-3-2-4-6-17/h2-10,13,15,23H,11-12,14,16H2,1H3/t23-,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human glucocorticoid receptor alpha isoform |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50167794

(1-(4-fluorophenyl)-4a-methyl-(4aS)-spiro[1,4,4a,5,...)Show SMILES C[C@]12Cc3cnn(c3C=C1CCC21OCCO1)-c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C19H19FN2O2/c1-18-11-13-12-21-22(16-4-2-15(20)3-5-16)17(13)10-14(18)6-7-19(18)23-8-9-24-19/h2-5,10,12H,6-9,11H2,1H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for human glucocorticoid receptor |
Bioorg Med Chem Lett 15: 2926-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.027
BindingDB Entry DOI: 10.7270/Q2X34X0Q |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217283

(CHEMBL1626887)Show SMILES C[C@@H](O)[C@@H]1[C@H]2CC(=C(N2C1=O)C(O)=O)c1ccc2C(=O)c3cc(C[N+]45CC[N+](CCO)(CC4)CC5)ccc3-c2c1 |c:6| Show InChI InChI=1S/C31H34N3O6/c1-18(36)27-26-16-23(28(31(39)40)32(26)30(27)38)20-3-5-22-24(15-20)21-4-2-19(14-25(21)29(22)37)17-34-9-6-33(7-10-34,8-11-34)12-13-35/h2-5,14-15,18,26-27,35-36H,6-13,16-17H2,1H3/q+1/p+1/t18-,26-,27-,33?,34?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217275

(CHEMBL1626955)Show SMILES C[C@@H](O)[C@@H]1[C@H]2CC(=C(N2C1=O)C(O)=O)c1ccc2C(=O)c3cc(C[N+]45CC[N+](C)(CC4)CC5)ccc3-c2c1 |c:6| Show InChI InChI=1S/C30H32N3O5/c1-17(34)26-25-15-22(27(30(37)38)31(25)29(26)36)19-4-6-21-23(14-19)20-5-3-18(13-24(20)28(21)35)16-33-10-7-32(2,8-11-33)9-12-33/h3-6,13-14,17,25-26,34H,7-12,15-16H2,1-2H3/q+1/p+1/t17-,25-,26-,32?,33?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217301

(CHEMBL1626950)Show SMILES CC[N+]12CC[N+](Cc3ccc-4c(c3)C(=O)c3ccc(cc-43)C3=C(N4[C@H](C3)[C@@H]([C@@H](C)O)C4=O)C(O)=O)(CC1)CC2 |t:24| Show InChI InChI=1S/C31H34N3O5/c1-3-33-8-11-34(12-9-33,13-10-33)17-19-4-6-21-24-15-20(5-7-22(24)29(36)25(21)14-19)23-16-26-27(18(2)35)30(37)32(26)28(23)31(38)39/h4-7,14-15,18,26-27,35H,3,8-13,16-17H2,1-2H3/q+1/p+1/t18-,26-,27-,33?,34?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 416 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217255

(CHEMBL1626901)Show SMILES C[C@@H](O)[C@@H]1[C@H]2[C@@H](C)C(=C(N2C1=O)C(O)=O)c1ccc2C(=O)c3cc(C[N+]45CC[N+](CC(=O)Nc6ccccn6)(CC4)CC5)ccc3-c2c1 |c:7| Show InChI InChI=1S/C37H37N5O6/c1-21-31(34(37(47)48)40-33(21)32(22(2)43)36(40)46)24-7-9-26-27(18-24)25-8-6-23(17-28(25)35(26)45)19-41-11-14-42(15-12-41,16-13-41)20-30(44)39-29-5-3-4-10-38-29/h3-10,17-18,21-22,32-33,43H,11-16,19-20H2,1-2H3/p+2/t21-,22+,32+,33+,41?,42?/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217287
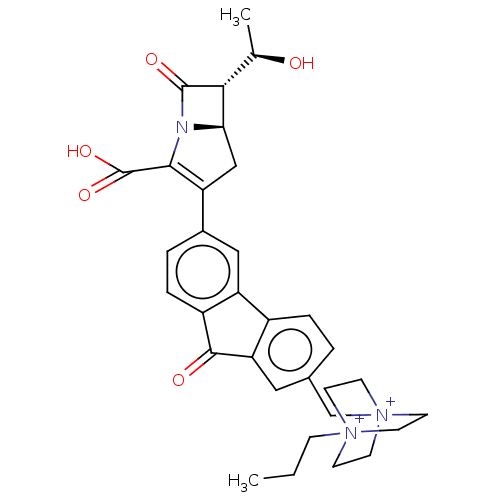
(CHEMBL1626933)Show SMILES CCC[N+]12CC[N+](Cc3ccc-4c(c3)C(=O)c3ccc(cc-43)C3=C(N4[C@H](C3)[C@@H]([C@@H](C)O)C4=O)C(O)=O)(CC1)CC2 |t:25| Show InChI InChI=1S/C32H36N3O5/c1-3-8-34-9-12-35(13-10-34,14-11-34)18-20-4-6-22-25-16-21(5-7-23(25)30(37)26(22)15-20)24-17-27-28(19(2)36)31(38)33(27)29(24)32(39)40/h4-7,15-16,19,27-28,36H,3,8-14,17-18H2,1-2H3/q+1/p+1/t19-,27-,28-,34?,35?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217263
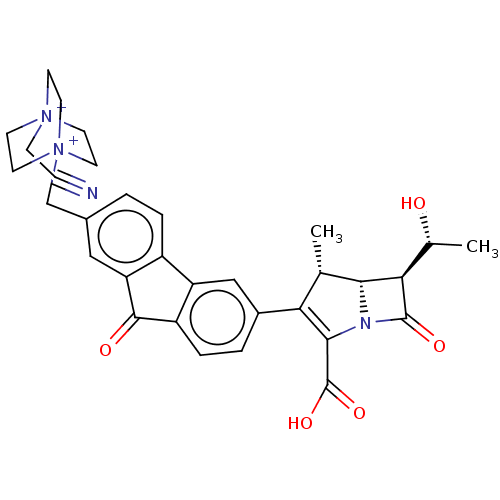
(CHEMBL1626899)Show SMILES C[C@@H](O)[C@@H]1[C@H]2[C@@H](C)C(=C(N2C1=O)C(O)=O)c1ccc2C(=O)c3cc(C[N+]45CC[N+](CC#N)(CC4)CC5)ccc3-c2c1 |c:7| Show InChI InChI=1S/C32H33N4O5/c1-18-26(29(32(40)41)34-28(18)27(19(2)37)31(34)39)21-4-6-23-24(16-21)22-5-3-20(15-25(22)30(23)38)17-36-12-9-35(8-7-33,10-13-36)11-14-36/h3-6,15-16,18-19,27-28,37H,8-14,17H2,1-2H3/q+1/p+1/t18-,19+,27+,28+,35?,36?/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217282

(CHEMBL1626947)Show SMILES C[C@@H](O)[C@@H]1[C@H]2CC(=C(N2C1=O)C(O)=O)c1ccc2C(=O)c3cc(C[N+]45CC[N+](CC(=O)Nc6ccccc6)(CC4)CC5)ccc3-c2c1 |c:6| Show InChI InChI=1S/C37H36N4O6/c1-22(42)33-31-19-28(34(37(46)47)39(31)36(33)45)24-8-10-27-29(18-24)26-9-7-23(17-30(26)35(27)44)20-40-11-14-41(15-12-40,16-13-40)21-32(43)38-25-5-3-2-4-6-25/h2-10,17-18,22,31,33,42H,11-16,19-21H2,1H3/p+2/t22-,31-,33-,40?,41?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 467 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217288

(CHEMBL1626948)Show SMILES C[C@@H](O)[C@@H]1[C@H]2CC(=C(N2C1=O)C(O)=O)c1ccc2C(=O)c3cc(C[N+]45CC[N+](CC#N)(CC4)CC5)ccc3-c2c1 |c:6| Show InChI InChI=1S/C31H31N4O5/c1-18(36)27-26-16-23(28(31(39)40)33(26)30(27)38)20-3-5-22-24(15-20)21-4-2-19(14-25(21)29(22)37)17-35-11-8-34(7-6-32,9-12-35)10-13-35/h2-5,14-15,18,26-27,36H,7-13,16-17H2,1H3/q+1/p+1/t18-,26-,27-,34?,35?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 474 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217291
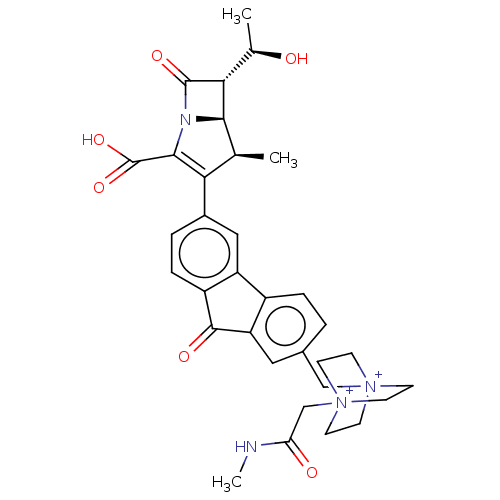
(CHEMBL1626970)Show SMILES CNC(=O)C[N+]12CC[N+](Cc3ccc-4c(c3)C(=O)c3ccc(cc-43)C3=C(N4[C@@H]([C@@H]([C@@H](C)O)C4=O)[C@H]3C)C(O)=O)(CC1)CC2 |t:27| Show InChI InChI=1S/C33H36N4O6/c1-18-27(30(33(42)43)35-29(18)28(19(2)38)32(35)41)21-5-7-23-24(15-21)22-6-4-20(14-25(22)31(23)40)16-36-8-11-37(12-9-36,13-10-36)17-26(39)34-3/h4-7,14-15,18-19,28-29,38H,8-13,16-17H2,1-3H3/p+2/t18-,19+,28+,29+,36?,37?/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217271

(CHEMBL1626903)Show SMILES C[C@@H](O)[C@@H]1[C@H]2[C@@H](C)C(=C(N2C1=O)C(O)=O)c1ccc2C(=O)c3cc(C[N+]45CC[N+](CCOc6ccccc6)(CC4)CC5)ccc3-c2c1 |c:7| Show InChI InChI=1S/C38H40N3O6/c1-23-32(35(38(45)46)39-34(23)33(24(2)42)37(39)44)26-9-11-29-30(21-26)28-10-8-25(20-31(28)36(29)43)22-41-15-12-40(13-16-41,14-17-41)18-19-47-27-6-4-3-5-7-27/h3-11,20-21,23-24,33-34,42H,12-19,22H2,1-2H3/q+1/p+1/t23-,24+,33+,34+,40?,41?/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 566 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |
Transglycosylase
(Staphylococcus aureus) | BDBM50217267

(CHEMBL432966 | L-741462)Show SMILES [Cl-].[H][C@]12CC(=C(N1C(=O)[C@]2([H])[C@@H](C)O)C([O-])=O)c1ccc2C(=O)c3cc(C[N+]45CC[N+](CC(N)=O)(CC4)CC5)ccc3-c2c1 |c:3| Show InChI InChI=1S/C31H32N4O6/c1-17(36)27-25-14-22(28(31(40)41)33(25)30(27)39)19-3-5-21-23(13-19)20-4-2-18(12-24(20)29(21)38)15-34-6-9-35(10-7-34,11-8-34)16-26(32)37/h2-5,12-13,17,25,27,36H,6-11,14-16H2,1H3,(H-2,32,37,40,41)/p+1/t17-,25-,27-,34?,35?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 573 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against PBP2a receptor by competition analysis with [3H]benzylpenicillin using cell membrane fractions from the MRSA COL strain. |
Bioorg Med Chem Lett 9: 3225-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KW5J62 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data