

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983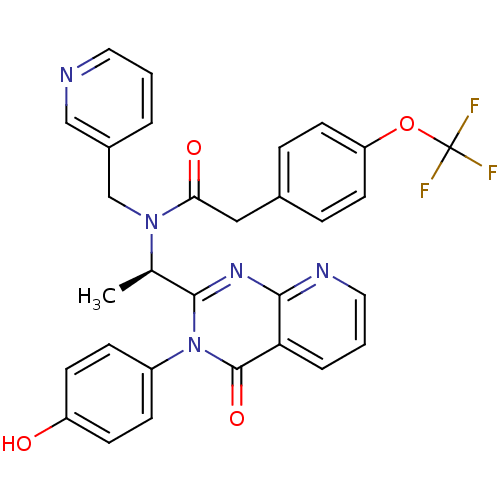 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate assessed as unbound inhibitor concentration required for half maximal enz... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate assessed as unbound inhibitor concentration required for half maximal ... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate assessed as residual enzyme activity after 2 to 10 mins by LC-MS/MS analy... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate assessed as residual enzyme activity after 2 to 10 mins by LC-MS/MS an... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by 10-fold dilution and ... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 30 mins followed by 10-fold dilution a... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50004704 ((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 90 mins followed by 10-fold dilution a... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181735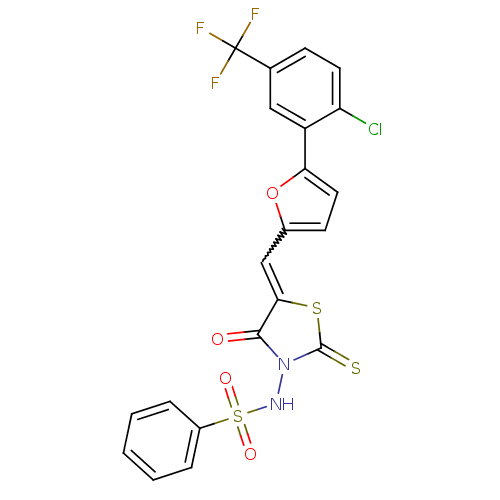 (5-(5-[2-chloro-5-(trifluoromethyl)phenyl]-furan-2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50004704 ((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by 10-fold dilution and ... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181696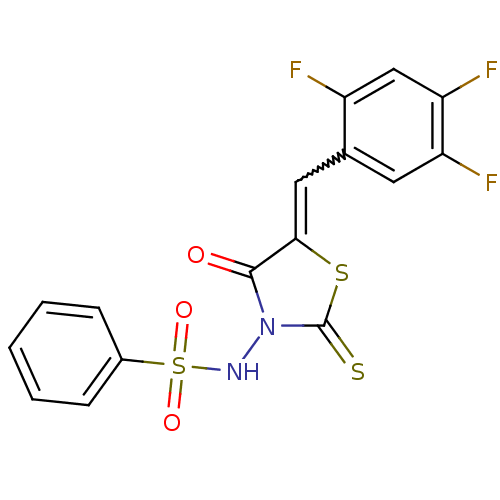 (5-(2,4,5-trifluorobenzylidene)-3-(benzenesulfonyla...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181720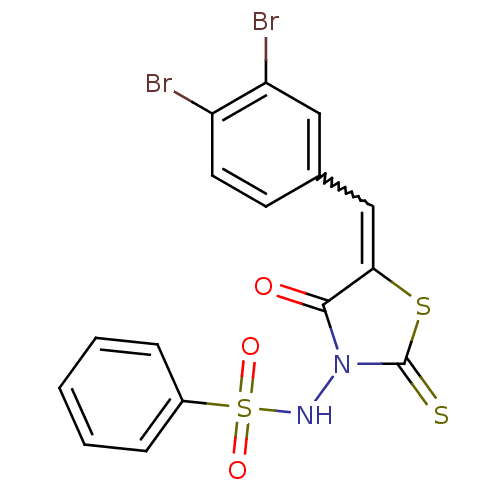 (5-(3,4-dibromobenzylidene)-3-(benzenesulfonylamino...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181684 (5-(2-methyl-3-phenyl-allylidene)-3-(benzenesulfony...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181685 (CHEMBL202580 | N-(5-(3,4-dichlorobenzylidene)-4-ox...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181692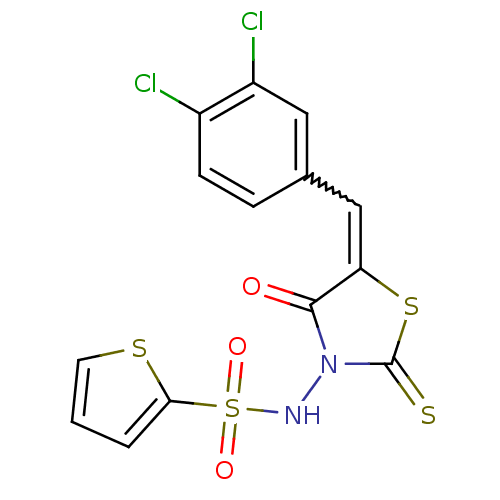 (5-(3,4-dichlorobenzylidene)-3-(2-thiophenesulfonyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181693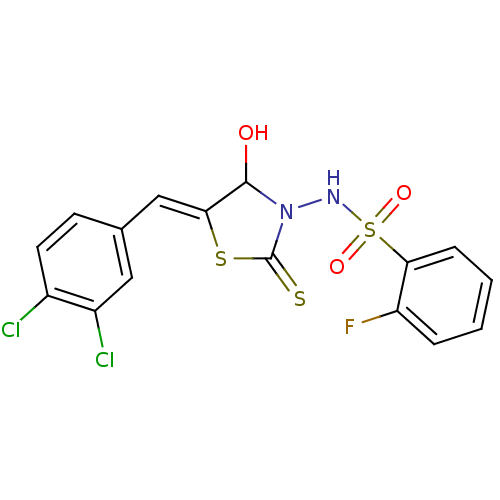 (5-(3,4-dichlorobenzylidene)-3-(2-fluorobenzenesulf...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181705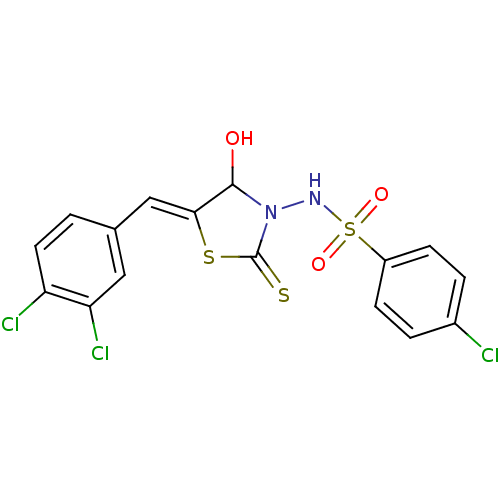 (5-(3,4-dichlorobenzylidene)-3-(4-chlorobenzenesulf...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181726 (5-(3,4-dichlorophenyl-1-ethylene)-3-(benzenesulfon...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181701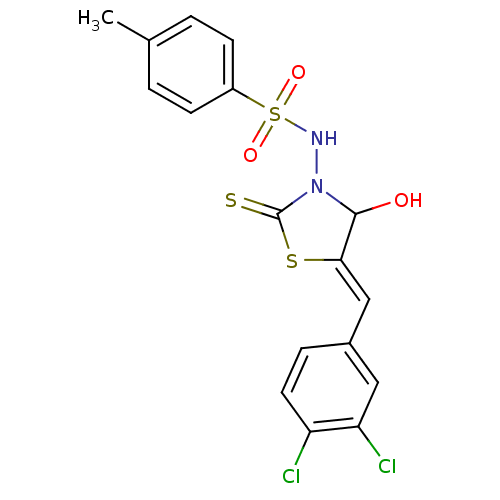 (5-(3,4-dichlorobenzylidene)-3-(4-methylbenzenesulf...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by 10-fold dilution and ... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181713 (5-(3,4-dichlorobenzylidene)-3-(butylsulfonylamino)...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181722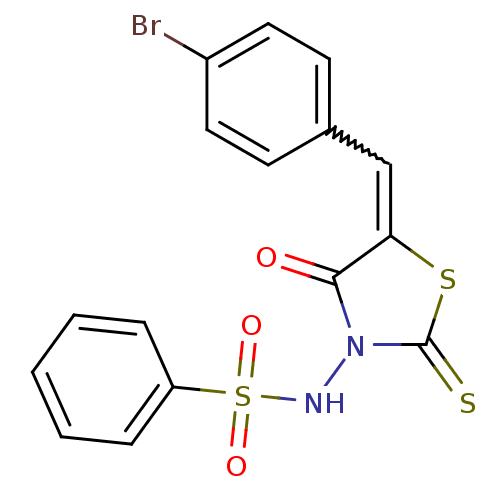 (5-(4-bromobenzylidene)-3-(benzenesulfonylamino)-4-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181700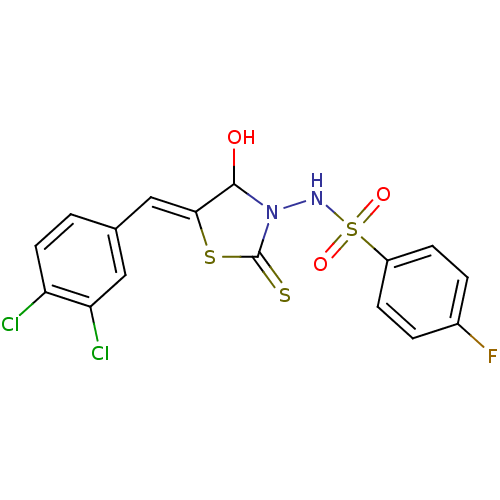 (5-(3,4-dichlorobenzylidene)-3-(4-fluorobenzenesulf...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181725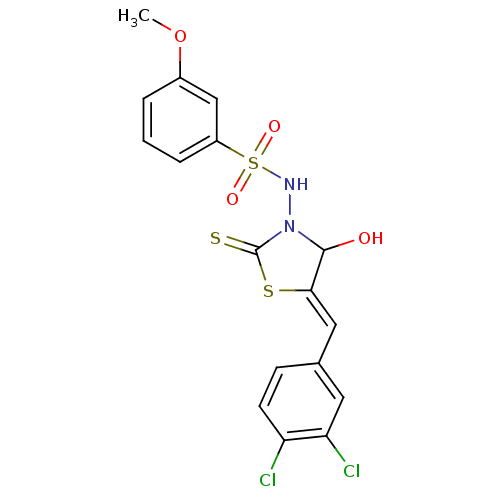 (5-(3,4-dichlorobenzylidene)-3-(3-methoxybenzenesul...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181686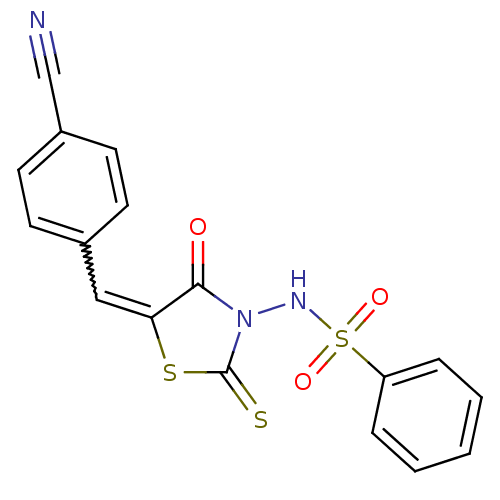 (5-(4-cyanobenzylidene)-3-(benzenesulfonylamino)-4-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181695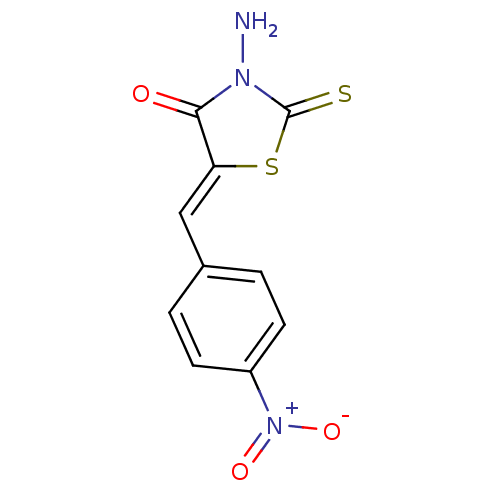 (5-(4-nitrobenzylidene)-3-amino-4-oxo-2-thionothiaz...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181717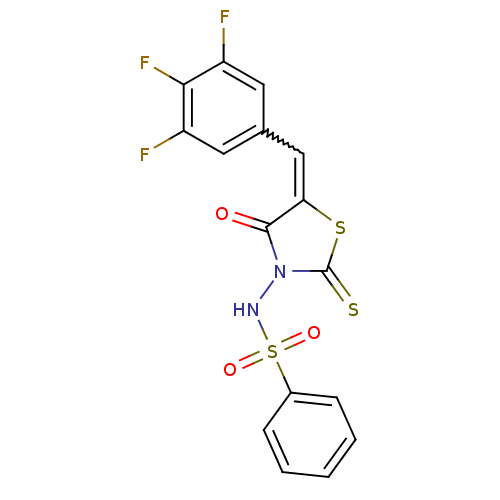 (5-(3,4,5-trifluorobenzylidene)-3-(benzenesulfonyla...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181714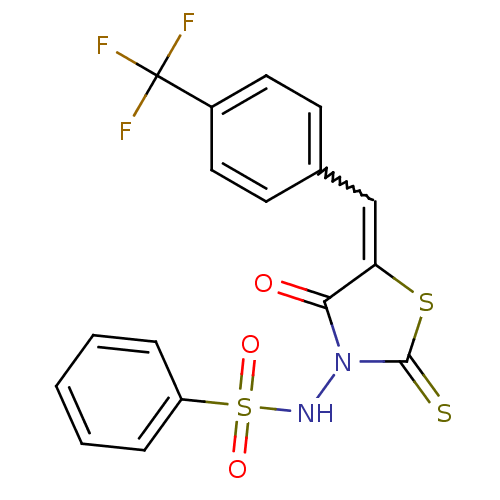 (5-(4-trifluoromethylbenzylidene)-3-(benzenesulfony...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181694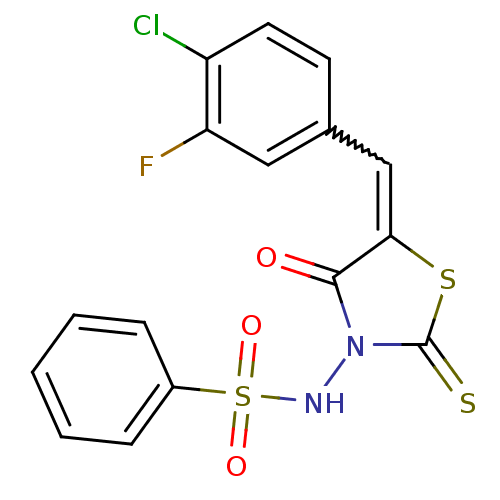 (5-(4-chloro-3-fluorobenzylidene)-3-(benzenesulfony...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181699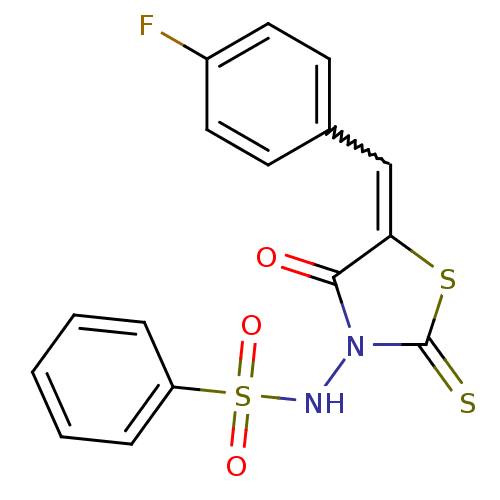 (5-(4-fluorobenzylidene)-3-(benzenesulfonylamino)-4...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181687 (5-(4-chlorobenzylidene)-3-(benzenesulfonylamino)-4...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181690 (5-(2,5-difluorobenzylidene)-3-(benzenesulfonylamin...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181704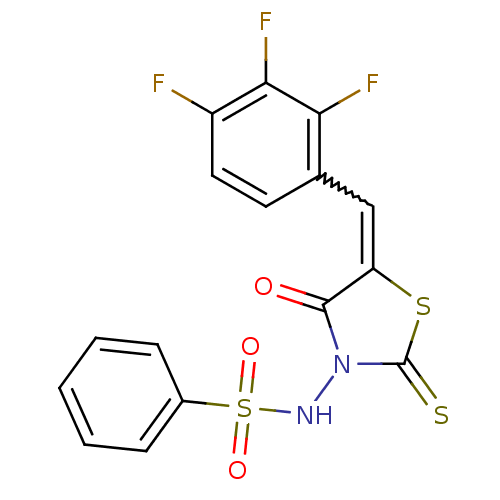 (5-(2,3,4-trifluorobenzylidene)-3-(benzenesulfonyla...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181710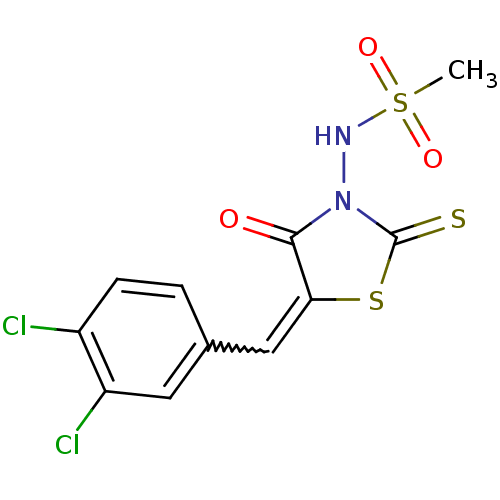 (5-(3,4-dichlorobenzylidene)-3-(methanesulfonylamin...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181706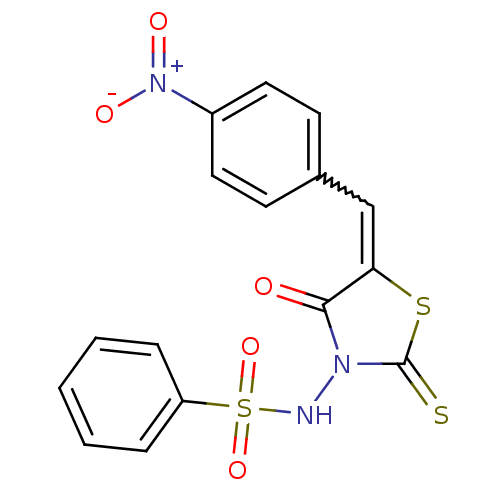 (5-(4-nitrobenzylidene)-3-(benzenesulfonylamino)-4-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50361983 (CHEMBL1939697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 30 mins followed by 10-fold dilution a... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50211114 ((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by 10-fold dilution and ... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50211114 ((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by substrate addition by... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181707 (5-(2-naphthylmethylene)-3-(benzenesulfonylamino)-4...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181689 (5-(2,4-difluorobenzylidene)-3-(benzenesulfonylamin...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181708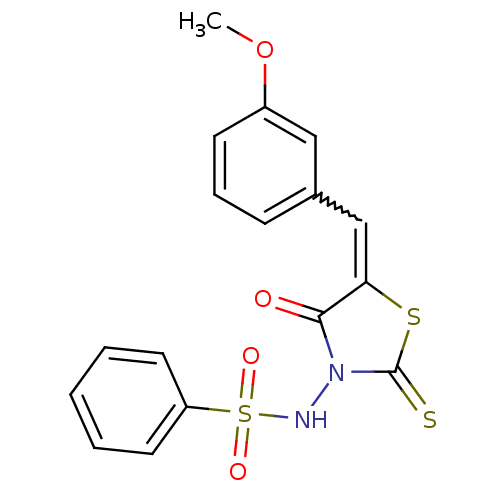 (5-(3-methoxybenzylidene)-3-(benzenesulfonylamino)-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181697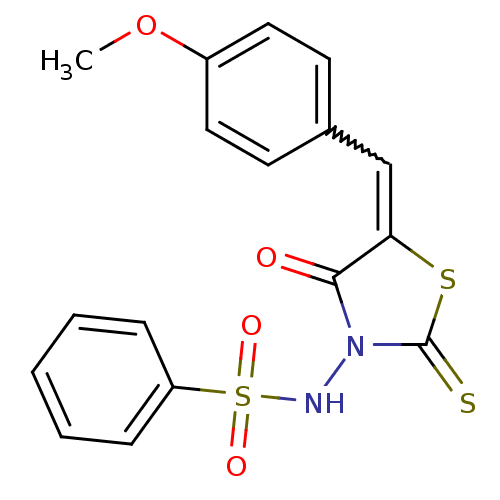 (5-(4-methoxybenzylidene)-3-(benzenesulfonylamino)-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50211114 ((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by 10-fold dilution and ... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50211114 ((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by substrate addition by... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181715 (5-(3,4-dichlorobenzylidene)-3-(4-methoxybenzenesul...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181691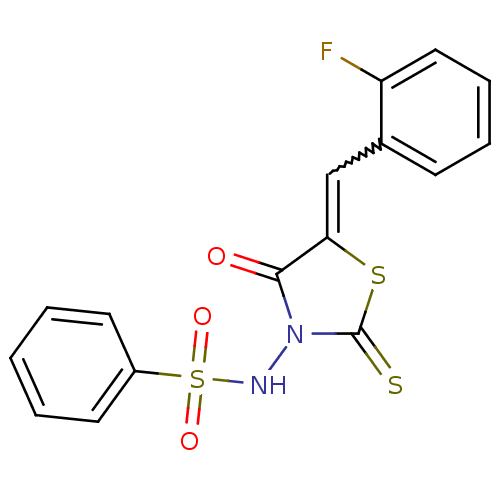 (5-(2-fluorobenzylidene)-3-(benzenesulfonylamino)-4...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181727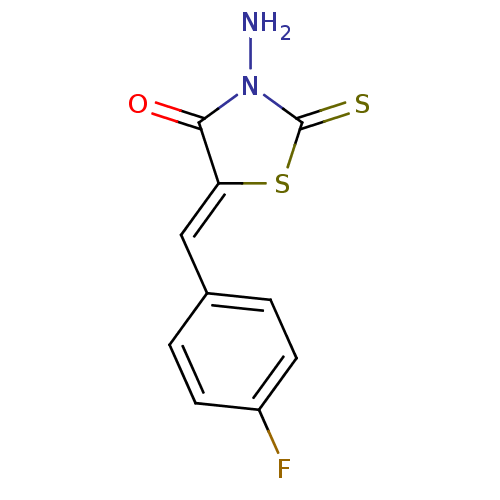 (5-(4-fluorobenzylidene)-3-amino-4-oxo-2-thionothia...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50211114 ((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by 10-fold dilution and ... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181709 (5-(4-cyanobenzylidene)-3-amino-4-oxo-2-thionothiaz...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50211114 ((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by 10-fold dilution and ... | Drug Metab Dispos 40: 1429-40 (2012) Article DOI: 10.1124/dmd.112.045708 BindingDB Entry DOI: 10.7270/Q23N254Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50181719 (5-(3,4-dimethylbenzylidene)-3-(benzenesulfonylamin...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5b RNA polymerase | J Med Chem 49: 1034-46 (2006) Article DOI: 10.1021/jm050859x BindingDB Entry DOI: 10.7270/Q2CJ8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 172 total ) | Next | Last >> |