 Found 199 hits with Last Name = 'merritt' and Initial = 'h'
Found 199 hits with Last Name = 'merritt' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM202656
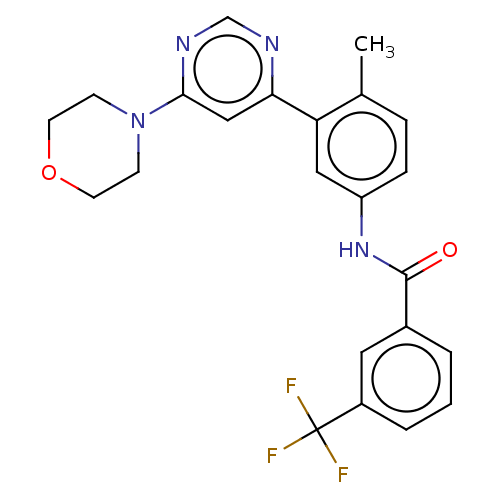
(US10245267, Example 1 | US10709712, Example 1 | US...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cc(ncn1)N1CCOCC1 Show InChI InChI=1S/C23H21F3N4O2/c1-15-5-6-18(29-22(31)16-3-2-4-17(11-16)23(24,25)26)12-19(15)20-13-21(28-14-27-20)30-7-9-32-10-8-30/h2-6,11-14H,7-10H2,1H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM202686

(US10245267, Example 31 | US10709712, Example 31 | ...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cc(nc(NCCO)n1)N1CCOCC1 Show InChI InChI=1S/C25H26F3N5O3/c1-16-5-6-19(30-23(35)17-3-2-4-18(13-17)25(26,27)28)14-20(16)21-15-22(33-8-11-36-12-9-33)32-24(31-21)29-7-10-34/h2-6,13-15,34H,7-12H2,1H3,(H,30,35)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM88120
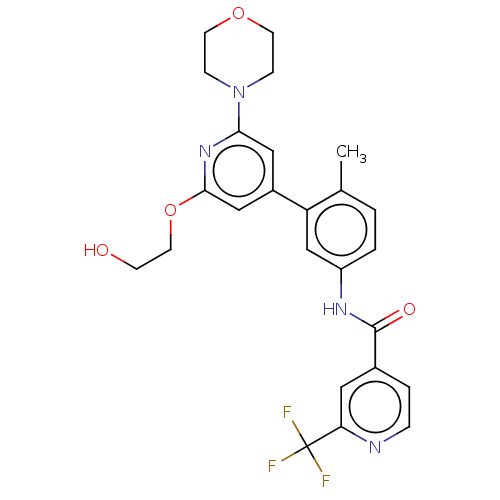
(US10245267, Example 1156 | US10709712, Example 115...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)C(F)(F)F)cc1-c1cc(OCCO)nc(c1)N1CCOCC1 Show InChI InChI=1S/C25H25F3N4O4/c1-16-2-3-19(30-24(34)17-4-5-29-21(12-17)25(26,27)28)15-20(16)18-13-22(32-6-9-35-10-7-32)31-23(14-18)36-11-8-33/h2-5,12-15,33H,6-11H2,1H3,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452149
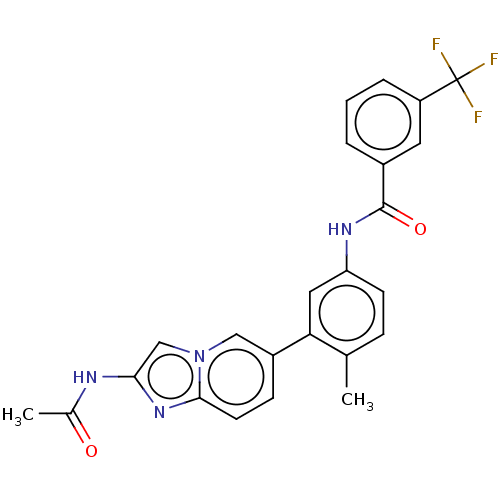
(CHEMBL4216073)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C24H19F3N4O2/c1-14-6-8-19(29-23(33)16-4-3-5-18(10-16)24(25,26)27)11-20(14)17-7-9-22-30-21(28-15(2)32)13-31(22)12-17/h3-13H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452150
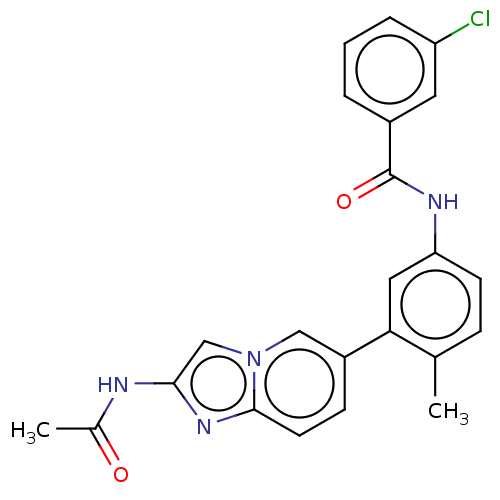
(CHEMBL4216386)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(Cl)c2)ccc1C Show InChI InChI=1S/C23H19ClN4O2/c1-14-6-8-19(26-23(30)16-4-3-5-18(24)10-16)11-20(14)17-7-9-22-27-21(25-15(2)29)13-28(22)12-17/h3-13H,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM87998

(US10245267, Example 1029 | US10709712, Example 103...)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cnc(O[C@H]2CCOC[C@H]2F)c(c1)N1CCOCC1 |r| Show InChI InChI=1S/C28H28F4N4O4/c1-17-22(13-21(15-33-17)35-26(37)18-3-2-4-20(11-18)28(30,31)32)19-12-24(36-6-9-38-10-7-36)27(34-14-19)40-25-5-8-39-16-23(25)29/h2-4,11-15,23,25H,5-10,16H2,1H3,(H,35,37)/t23-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452152
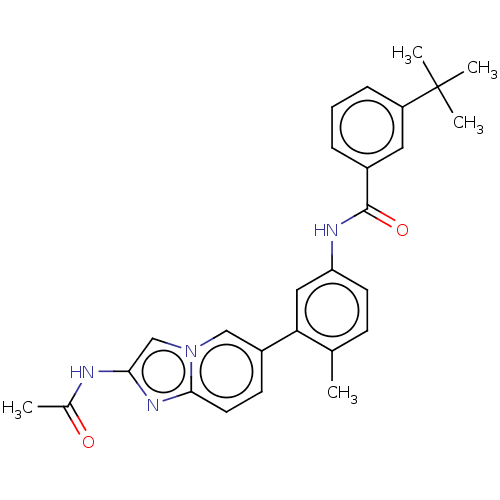
(CHEMBL4217462)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(C)(C)C)ccc1C Show InChI InChI=1S/C27H28N4O2/c1-17-9-11-22(29-26(33)19-7-6-8-21(13-19)27(3,4)5)14-23(17)20-10-12-25-30-24(28-18(2)32)16-31(25)15-20/h6-16H,1-5H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM84365

(US10245267, Example 636 | US10709712, Example 636 ...)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cc(N2CCOCC2)c(OC2CCOCC2)nn1 Show InChI InChI=1S/C27H28F3N5O4/c1-17-22(14-20(16-31-17)32-25(36)18-3-2-4-19(13-18)27(28,29)30)23-15-24(35-7-11-38-12-8-35)26(34-33-23)39-21-5-9-37-10-6-21/h2-4,13-16,21H,5-12H2,1H3,(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM202784

(US10245267, Example 131 | US10709712, Example 131 ...)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cnc(OC2CCOCC2)c(c1)N1CCOCC1 Show InChI InChI=1S/C28H29F3N4O4/c1-18-24(15-22(17-32-18)34-26(36)19-3-2-4-21(13-19)28(29,30)31)20-14-25(35-7-11-38-12-8-35)27(33-16-20)39-23-5-9-37-10-6-23/h2-4,13-17,23H,5-12H2,1H3,(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full-length BRAF (unknown origin) |
J Med Chem 60: 4869-4881 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01862
BindingDB Entry DOI: 10.7270/Q2F191ZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM202784

(US10245267, Example 131 | US10709712, Example 131 ...)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cnc(OC2CCOCC2)c(c1)N1CCOCC1 Show InChI InChI=1S/C28H29F3N4O4/c1-18-24(15-22(17-32-18)34-26(36)19-3-2-4-21(13-19)28(29,30)31)20-14-25(35-7-11-38-12-8-35)27(33-16-20)39-23-5-9-37-10-6-23/h2-4,13-17,23H,5-12H2,1H3,(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM88120

(US10245267, Example 1156 | US10709712, Example 115...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)C(F)(F)F)cc1-c1cc(OCCO)nc(c1)N1CCOCC1 Show InChI InChI=1S/C25H25F3N4O4/c1-16-2-3-19(30-24(34)17-4-5-29-21(12-17)25(26,27)28)15-20(16)18-13-22(32-6-9-35-10-7-32)31-23(14-18)36-11-8-33/h2-5,12-15,33H,6-11H2,1H3,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full-length BRAF (unknown origin) |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50510891
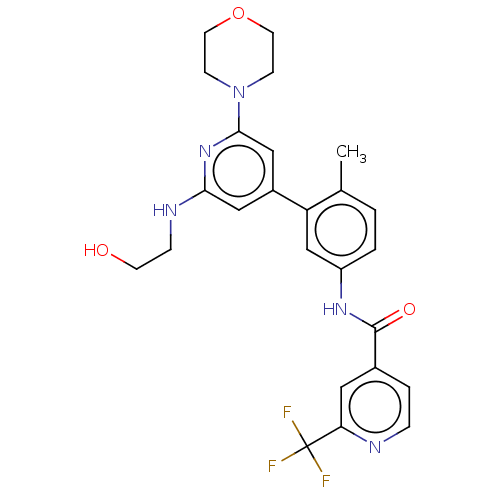
(CHEMBL4455996)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)C(F)(F)F)cc1-c1cc(NCCO)nc(c1)N1CCOCC1 Show InChI InChI=1S/C25H26F3N5O3/c1-16-2-3-19(31-24(35)17-4-5-29-21(12-17)25(26,27)28)15-20(16)18-13-22(30-6-9-34)32-23(14-18)33-7-10-36-11-8-33/h2-5,12-15,34H,6-11H2,1H3,(H,30,32)(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM88006
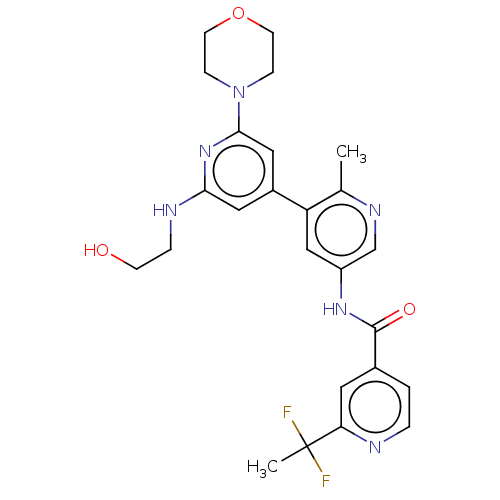
(US10245267, Example 1041 | US10709712, Example 104...)Show SMILES Cc1ncc(NC(=O)c2ccnc(c2)C(C)(F)F)cc1-c1cc(NCCO)nc(c1)N1CCOCC1 Show InChI InChI=1S/C25H28F2N6O3/c1-16-20(18-12-22(29-5-8-34)32-23(13-18)33-6-9-36-10-7-33)14-19(15-30-16)31-24(35)17-3-4-28-21(11-17)25(2,26)27/h3-4,11-15,34H,5-10H2,1-2H3,(H,29,32)(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50510890
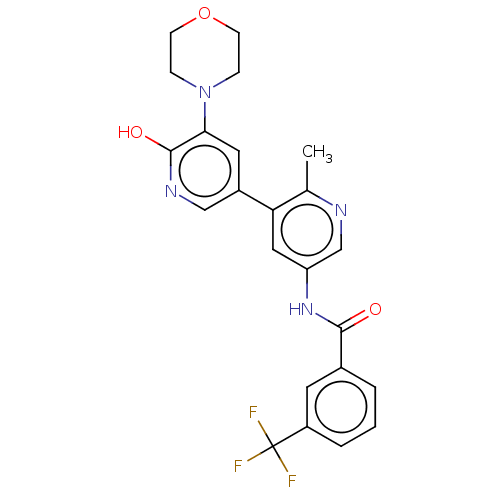
(CHEMBL4593446)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1cnc(O)c(c1)N1CCOCC1 Show InChI InChI=1S/C23H21F3N4O3/c1-14-19(16-10-20(22(32)28-12-16)30-5-7-33-8-6-30)11-18(13-27-14)29-21(31)15-3-2-4-17(9-15)23(24,25)26/h2-4,9-13H,5-8H2,1H3,(H,28,32)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452147
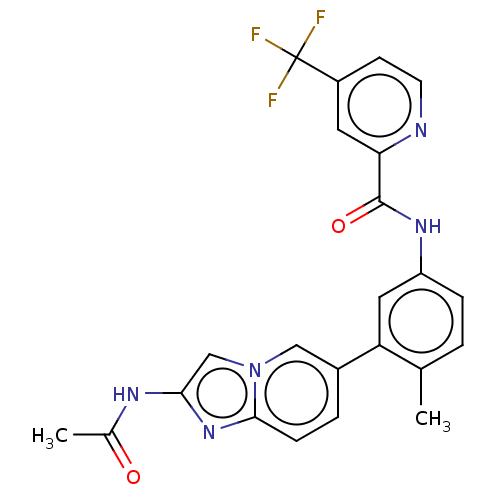
(CHEMBL4204192)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cc(ccn2)C(F)(F)F)ccc1C Show InChI InChI=1S/C23H18F3N5O2/c1-13-3-5-17(29-22(33)19-9-16(7-8-27-19)23(24,25)26)10-18(13)15-4-6-21-30-20(28-14(2)32)12-31(21)11-15/h3-12H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM202839

(US10245267, Example 640 | US10709712, Example 191 ...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)C(C)(C)C#N)cc1-c1cc(N2CCOCC2)c(=O)n(C)n1 Show InChI InChI=1S/C26H28N6O3/c1-17-5-6-19(29-24(33)18-7-8-28-23(13-18)26(2,3)16-27)14-20(17)21-15-22(25(34)31(4)30-21)32-9-11-35-12-10-32/h5-8,13-15H,9-12H2,1-4H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50510889
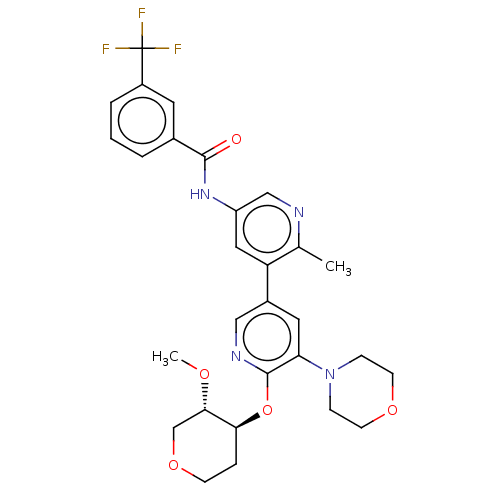
(CHEMBL4475855)Show SMILES CO[C@H]1COCC[C@@H]1Oc1ncc(cc1N1CCOCC1)-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)cnc1C |r| Show InChI InChI=1S/C29H31F3N4O5/c1-18-23(14-22(16-33-18)35-27(37)19-4-3-5-21(12-19)29(30,31)32)20-13-24(36-7-10-39-11-8-36)28(34-15-20)41-25-6-9-40-17-26(25)38-2/h3-5,12-16,25-26H,6-11,17H2,1-2H3,(H,35,37)/t25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CRAF Y340E/Y341E mutant (unknown origin) using human MEK1 K97R mutant as substrate pretreated for 30 mins followed by substrate additio... |
J Med Chem 63: 2013-2027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00161
BindingDB Entry DOI: 10.7270/Q20G3PG4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50452152

(CHEMBL4217462)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(C)(C)C)ccc1C Show InChI InChI=1S/C27H28N4O2/c1-17-9-11-22(29-26(33)19-7-6-8-21(13-19)27(3,4)5)14-23(17)20-10-12-25-30-24(28-18(2)32)16-31(25)15-20/h6-16H,1-5H3,(H,28,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P38 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50452151
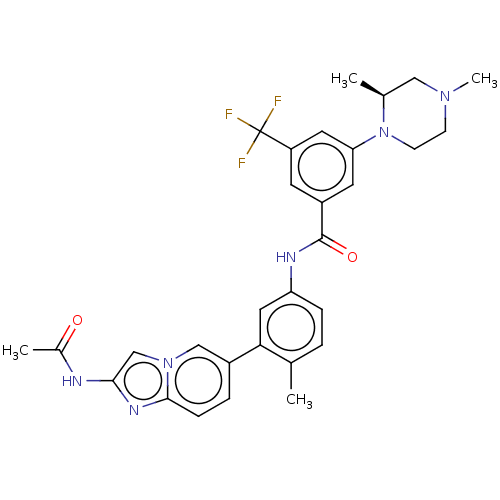
(CHEMBL4208527)Show SMILES C[C@H]1CN(C)CCN1c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(c1)-c1ccc2nc(NC(C)=O)cn2c1 |r| Show InChI InChI=1S/C30H31F3N6O2/c1-18-5-7-24(14-26(18)21-6-8-28-36-27(34-20(3)40)17-38(28)16-21)35-29(41)22-11-23(30(31,32)33)13-25(12-22)39-10-9-37(4)15-19(39)2/h5-8,11-14,16-17,19H,9-10,15H2,1-4H3,(H,34,40)(H,35,41)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P38 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721
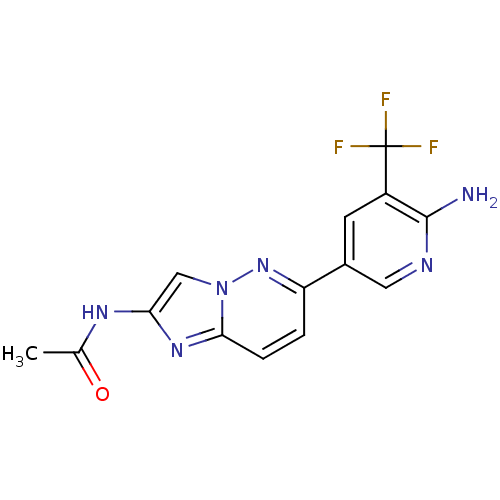
(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721

(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140266
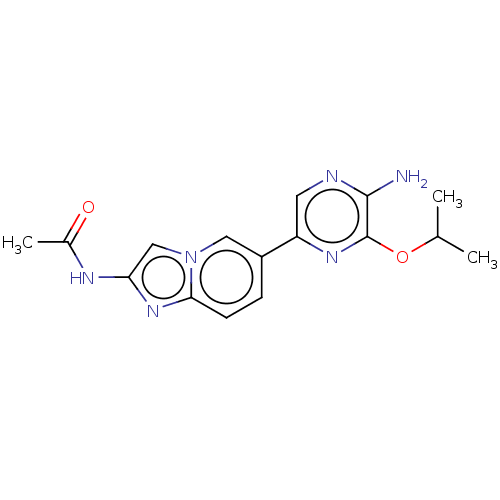
(CHEMBL3753366)Show InChI InChI=1S/C16H18N6O2/c1-9(2)24-16-15(17)18-6-12(20-16)11-4-5-14-21-13(19-10(3)23)8-22(14)7-11/h4-9H,1-3H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380371
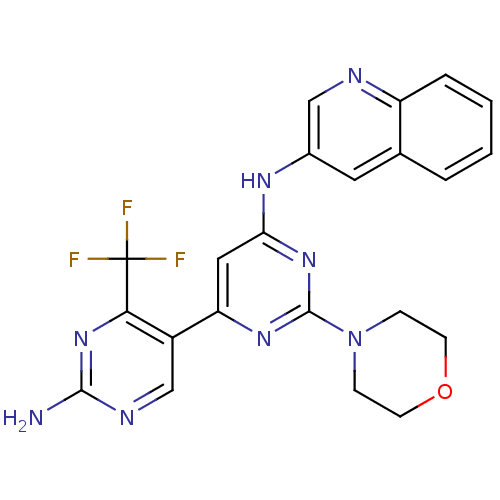
(CHEMBL2017970)Show SMILES Nc1ncc(-c2cc(Nc3cnc4ccccc4c3)nc(n2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C22H19F3N8O/c23-22(24,25)19-15(12-28-20(26)32-19)17-10-18(31-21(30-17)33-5-7-34-8-6-33)29-14-9-13-3-1-2-4-16(13)27-11-14/h1-4,9-12H,5-8H2,(H2,26,28,32)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380366
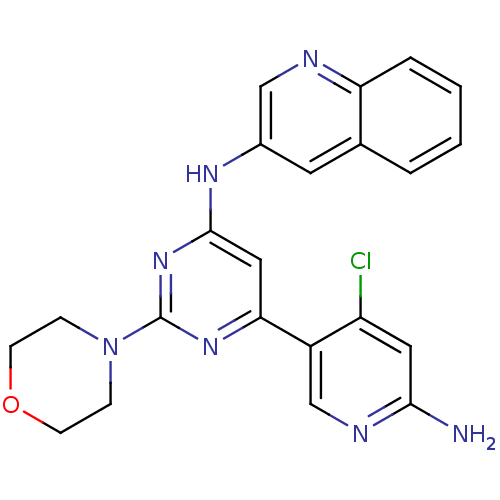
(CHEMBL2017965)Show SMILES Nc1cc(Cl)c(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H20ClN7O/c23-17-10-20(24)26-13-16(17)19-11-21(29-22(28-19)30-5-7-31-8-6-30)27-15-9-14-3-1-2-4-18(14)25-12-15/h1-4,9-13H,5-8H2,(H2,24,26)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380369

(CHEMBL2017968)Show SMILES Nc1ncc(c(N)n1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H21N9O/c22-19-15(12-25-20(23)29-19)17-10-18(28-21(27-17)30-5-7-31-8-6-30)26-14-9-13-3-1-2-4-16(13)24-11-14/h1-4,9-12H,5-8H2,(H,26,27,28)(H4,22,23,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140267
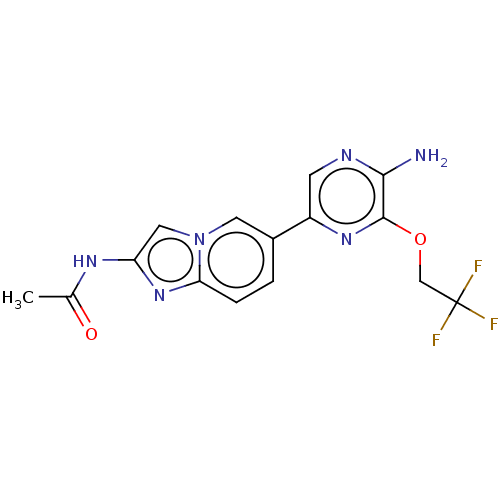
(CHEMBL3752019)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(OCC(F)(F)F)n1 Show InChI InChI=1S/C15H13F3N6O2/c1-8(25)21-11-6-24-5-9(2-3-12(24)23-11)10-4-20-13(19)14(22-10)26-7-15(16,17)18/h2-6H,7H2,1H3,(H2,19,20)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140269
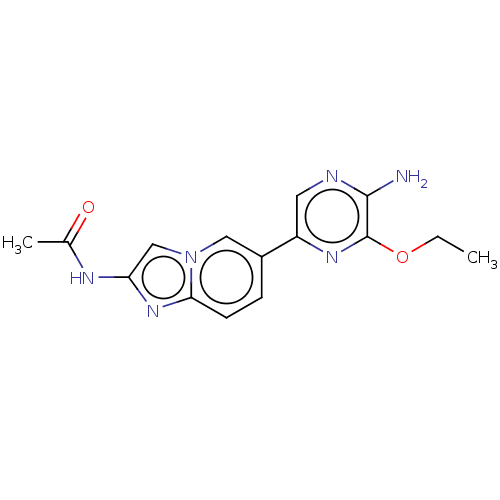
(CHEMBL3752653)Show InChI InChI=1S/C15H16N6O2/c1-3-23-15-14(16)17-6-11(19-15)10-4-5-13-20-12(18-9(2)22)8-21(13)7-10/h4-8H,3H2,1-2H3,(H2,16,17)(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140270
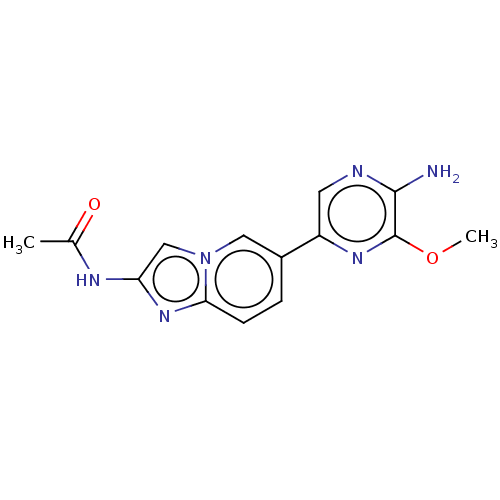
(CHEMBL3752503)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-11-7-20-6-9(3-4-12(20)19-11)10-5-16-13(15)14(18-10)22-2/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452151

(CHEMBL4208527)Show SMILES C[C@H]1CN(C)CCN1c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(c1)-c1ccc2nc(NC(C)=O)cn2c1 |r| Show InChI InChI=1S/C30H31F3N6O2/c1-18-5-7-24(14-26(18)21-6-8-28-36-27(34-20(3)40)17-38(28)16-21)35-29(41)22-11-23(30(31,32)33)13-25(12-22)39-10-9-37(4)15-19(39)2/h5-8,11-14,16-17,19H,9-10,15H2,1-4H3,(H,34,40)(H,35,41)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140265
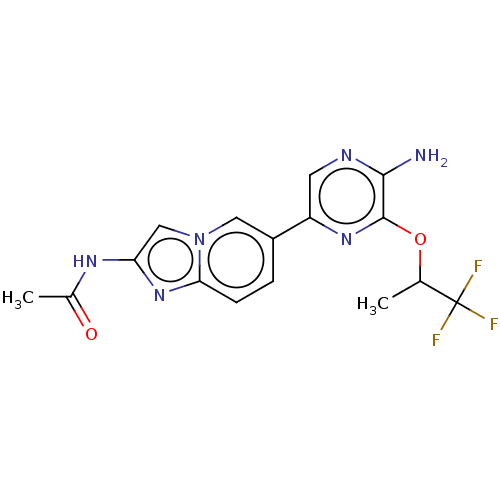
(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711
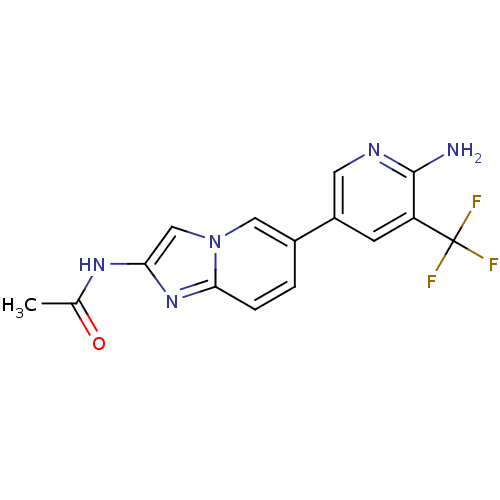
(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1alpha-phosphotidylinositol by luminescence assay |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
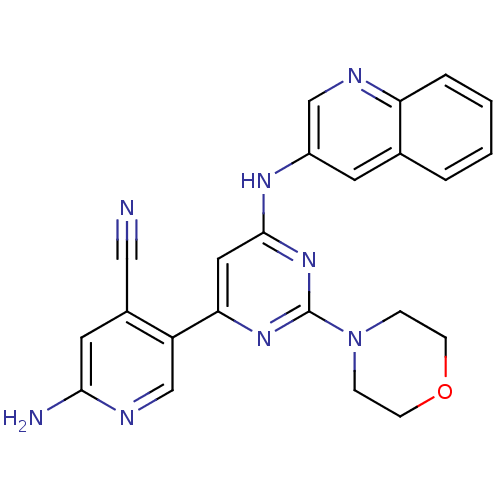
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380367
(CHEMBL2017966)Show SMILES Nc1cc(C#N)c(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C23H20N8O/c24-12-16-10-21(25)27-14-18(16)20-11-22(30-23(29-20)31-5-7-32-8-6-31)28-17-9-15-3-1-2-4-19(15)26-13-17/h1-4,9-11,13-14H,5-8H2,(H2,25,27)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
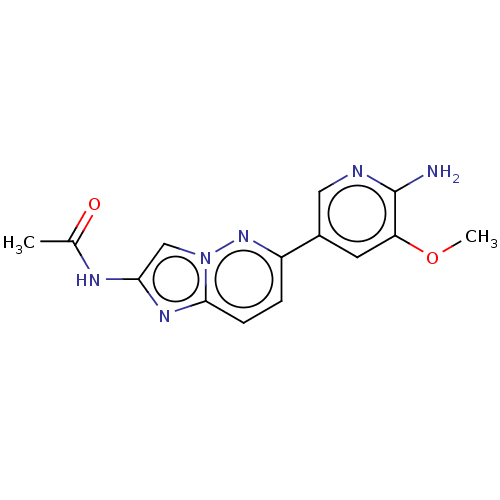
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140273
(CHEMBL3752760)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-12-7-20-13(18-12)4-3-10(19-20)9-5-11(22-2)14(15)16-6-9/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452154

(CHEMBL4205266)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)-c2ccc3nc(NC(C)=O)cn3c2)CC1 Show InChI InChI=1S/C30H31F3N6O2/c1-19-4-8-24(15-25(19)22-7-9-28-36-27(34-20(2)40)18-39(28)17-22)35-29(41)21-5-6-23(26(14-21)30(31,32)33)16-38-12-10-37(3)11-13-38/h4-9,14-15,17-18H,10-13,16H2,1-3H3,(H,34,40)(H,35,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
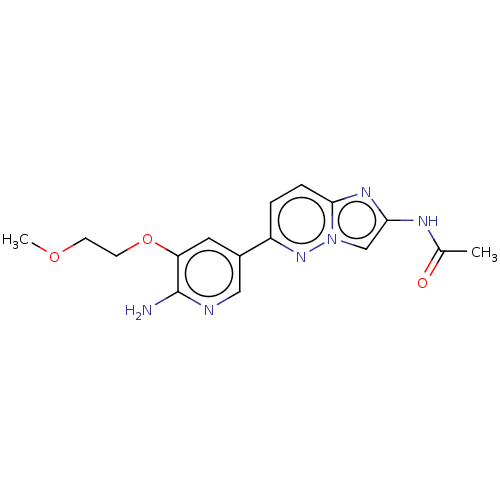
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140271
(CHEMBL3751961)Show InChI InChI=1S/C16H18N6O3/c1-10(23)19-14-9-22-15(20-14)4-3-12(21-22)11-7-13(16(17)18-8-11)25-6-5-24-2/h3-4,7-9H,5-6H2,1-2H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452148
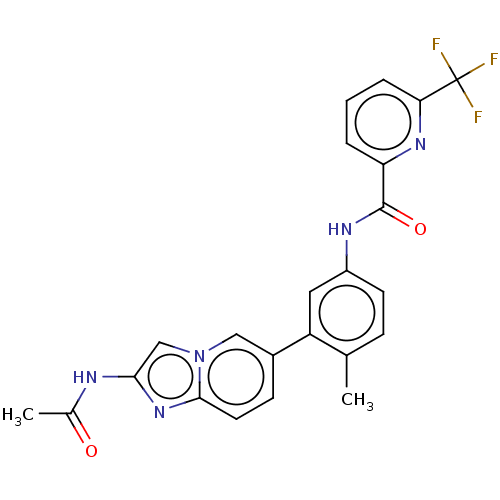
(CHEMBL4209163)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(n2)C(F)(F)F)ccc1C Show InChI InChI=1S/C23H18F3N5O2/c1-13-6-8-16(28-22(33)18-4-3-5-19(29-18)23(24,25)26)10-17(13)15-7-9-21-30-20(27-14(2)32)12-31(21)11-15/h3-12H,1-2H3,(H,27,32)(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50331592

(3-(6-(6-methoxypyridin-3-ylamino)-2-morpholinopyri...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cccc(O)c2)cn1 Show InChI InChI=1S/C20H21N5O3/c1-27-19-6-5-15(13-21-19)22-18-12-17(14-3-2-4-16(26)11-14)23-20(24-18)25-7-9-28-10-8-25/h2-6,11-13,26H,7-10H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50140265

(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380370

(CHEMBL2017969)Show SMILES Nc1ncc(-c2cc(Nc3cnc4ccccc4c3)nc(n2)N2CCOCC2)c(=O)[nH]1 Show InChI InChI=1S/C21H20N8O2/c22-20-24-12-15(19(30)28-20)17-10-18(27-21(26-17)29-5-7-31-8-6-29)25-14-9-13-3-1-2-4-16(13)23-11-14/h1-4,9-12H,5-8H2,(H,25,26,27)(H3,22,24,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439726
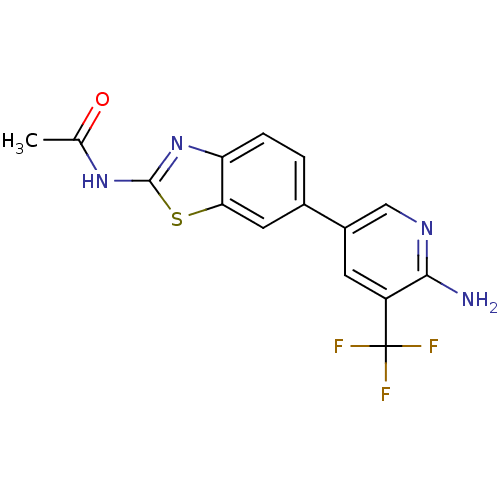
(CHEMBL2418948)Show SMILES CC(=O)Nc1nc2ccc(cc2s1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H11F3N4OS/c1-7(23)21-14-22-11-3-2-8(5-12(11)24-14)9-4-10(15(16,17)18)13(19)20-6-9/h2-6H,1H3,(H2,19,20)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50140265

(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140268

(CHEMBL3753085)Show InChI InChI=1S/C16H18N6O3/c1-10(23)19-13-9-22-8-11(3-4-14(22)21-13)12-7-18-15(17)16(20-12)25-6-5-24-2/h3-4,7-9H,5-6H2,1-2H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50452149

(CHEMBL4216073)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C24H19F3N4O2/c1-14-6-8-19(29-23(33)16-4-3-5-18(10-16)24(25,26)27)11-20(14)17-7-9-22-30-21(28-15(2)32)13-31(22)12-17/h3-13H,1-2H3,(H,28,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380372
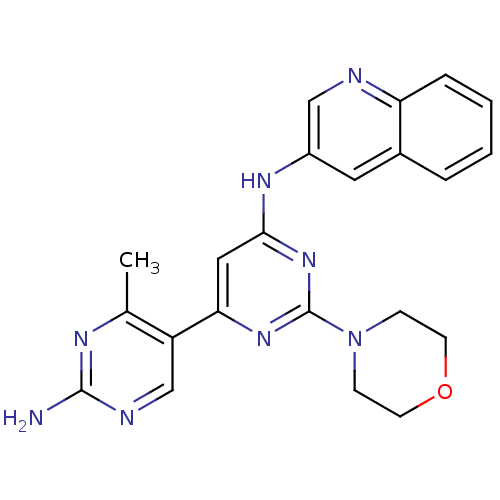
(CHEMBL2016592)Show SMILES Cc1nc(N)ncc1-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H22N8O/c1-14-17(13-25-21(23)26-14)19-11-20(29-22(28-19)30-6-8-31-9-7-30)27-16-10-15-4-2-3-5-18(15)24-12-16/h2-5,10-13H,6-9H2,1H3,(H2,23,25,26)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using 1alpha-phosphotidylinositol by luminescence assay |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140272
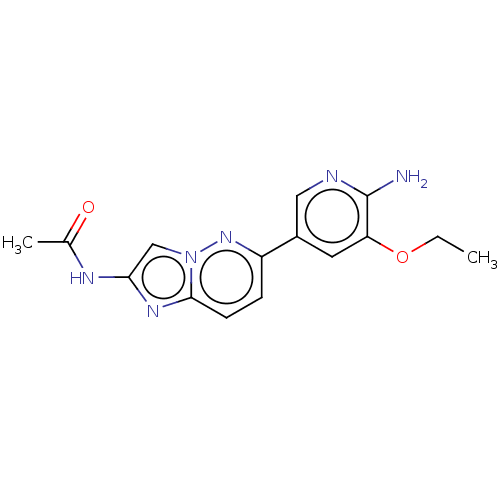
(CHEMBL3754572)Show InChI InChI=1S/C15H16N6O2/c1-3-23-12-6-10(7-17-15(12)16)11-4-5-14-19-13(18-9(2)22)8-21(14)20-11/h4-8H,3H2,1-2H3,(H2,16,17)(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50452154

(CHEMBL4205266)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)-c2ccc3nc(NC(C)=O)cn3c2)CC1 Show InChI InChI=1S/C30H31F3N6O2/c1-19-4-8-24(15-25(19)22-7-9-28-36-27(34-20(2)40)18-39(28)17-22)35-29(41)21-5-6-23(26(14-21)30(31,32)33)16-38-12-10-37(3)11-13-38/h4-9,14-15,17-18H,10-13,16H2,1-3H3,(H,34,40)(H,35,41) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P38 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140275

(CHEMBL3752775)Show InChI InChI=1S/C15H15N5O2/c1-9(21)18-13-8-20-7-10(3-4-14(20)19-13)11-5-12(22-2)15(16)17-6-11/h3-8H,1-2H3,(H2,16,17)(H,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data